INTRODUCTION
Internationally, respiratory syncytial virus (RSV) is the leading cause of lower respiratory tract disease during infancy and is responsible for 50–80% of hospitalizations for bronchiolitis [Reference Smyth and Openshaw1]. In New Zealand (NZ) bronchiolitis admission rates are increasing. In 1998 the rate of bronchiolitis admissions for infants aged <12 months was estimated at 58/1000 [Reference Vogel, Lennon and Harding2], roughly double that of North American and European infants [Reference Shay3, Reference Simoes and Carbonell-Estrany4]. More recently, NZ Health Information Service public hospital discharge data for 2003–2005 show bronchiolitis rates for infants aged <12 months have increased to 72/1000 [5].
Major risk factors for RSV bronchiolitis hospitalization include: pre-term delivery, severe underlying cardiac, respiratory or neuromuscular disease, and immunodeficiency [Reference Welliver6]. However, an audit of NZ bronchiolitis hospitalizations revealed that increased admission rates were unaccompanied by proportionate increases in these risk factors [Reference Vogel, Lennon and Harding2, Reference Vogel7]. Therefore, in order to more accurately assess risk factors for hospitalization in NZ, we undertook a single centre, hospital-based study of RSV bronchiolitis during three consecutive RSV epidemic seasons (2003–2005) in Wellington, NZ.
METHODS
Setting and subjects
Wellington Hospital provides the city's sole in-patient paediatric services. Eligible cases were infants aged <24 months in hospital Monday–Friday with community-acquired bronchiolitis during three consecutive RSV epidemic seasons (June/July to October 2003–2005). A diagnosis of bronchiolitis was based upon coryzal symptoms followed by signs of respiratory distress and fine, inspiratory crackles on auscultation [Reference Barry8]. Hospital admission guidelines included one or more of the following: (i) respiratory distress (e.g. tachynpnoea, chest recession), (ii) apnoea, (iii) inability to feed, (iv) pulse oximetry <92% in air, (v) underlying chronic medical conditions such as cardiopulmonary diseases, immunodeficiency or neuromuscular disorders and (vi) adverse social circumstances from a lack of transport or telephone [Reference Dawson9]. The Central Regional Ethics Committee approved the study and written, informed consent was gained from the infant's parent or guardian.
Demographic and clinical data
Demographic and clinical data were collected during hospitalization. Gender, ethnicity, gestational age, birth weight, age and weight at admission, breastfeeding history, tobacco smoke exposure and underlying chronic medical disorders were recorded by a nurse-administered 25-item questionnaire (English or Pacific languages). Ethnicity was determined using NZ Census data methodology that prioritizes ethnicity of those identifying with multiple ethnic groups to a single ethnic category as follows: Māori>Pacific>Other>NZ European/Pakeha (Caucasian) [10].
The severity index score took oxygen requirement as the best single measure of illness severity in hospitalized infants with bronchiolitis [Reference McIntosh, De Silva and Oates11]. Infants requiring assisted ventilation or continuous positive airway pressure (CPAP) were classified as severe, those requiring oxygen supplementation as moderate, and those hospitalized but not requiring oxygen as mild. Length of hospitalization was recorded as 0–24 h=1 day, 25–48 h=2 days and so on.
The New Zealand Deprivation Index (NZDep2001) was used as a proxy measure for socioeconomic status and was determined from the infant's address. NZDep2001 used pooled 2001 census data for nine dimensions of material and social status, including household crowding, to measure socioeconomic status at a neighbourhood level. A score ranging from 1 to 10 was allocated to each neighbourhood, decile 1 representing the least deprived 10% of neighbourhoods and decile 10 the most deprived 10% [Reference Salmond and Crampton12].
The Wellington Women's Hospital Perinatal Information Management System database allowed comparisons to be made between subject and infant birth populations from the same region. Gender, month of birth, gestational age, birth weight, singleton pregnancy, ethnicity, tobacco smoke exposure during pregnancy and NZDep2001 scores were obtained from the database for all live hospital births in the Wellington region for 2003–2005.
Management of cases
Infants were managed by continuous monitoring of oxygen saturation by pulse oximetry and, if required, supplemental oxygen maintained oxygen saturation >92% [Reference Vogel7]. Infants not tolerating oral fluids received intravenous fluids or milk via a nasogastric tube. CPAP and intravenous fluids were administered when infants required more than 60% inspired oxygen, while assisted ventilation was instituted for recurrent apnoea, increasing hypoxaemia or exhaustion. No drugs were used routinely. Discharge followed re-establishment of normal feeding without supplemental oxygen and absence of fever.
Sample collection and laboratory methods
Nasopharyngeal aspirates were performed routinely on infants admitted with bronchiolitis. The Hospital's Diagnostic Laboratory conducted RSV antigen testing by direct immunofluorescence assay (RSV Imagen, DakoCytomation, Cambridgeshire, UK) within 2 h of sample collection. An aliquot from each specimen was taken immediately to the Malaghan Institute, where RNA was extracted and viral subtyping conducted by RT–PCR and nested PCR [Reference Matheson13].
Statistical analysis
Data were analysed using SAS version 9.1 (SAS Institute, Cary, NC, USA) and Stata statistical software (release 8.2, Stata Corporation, College Station, TX, USA). Analyses of RSV incidence were conducted using Poisson regression to estimate incidence rate ratios [Reference Rothman and Greenland14] initially with univariate analyses, then with multiple regression analyses including variables for birth weight, ethnicity, gestation, NZDep2001 score and passive tobacco smoke exposure. Analyses of factors that affected severity in RSV-positive children were conducted using logistic regression to estimate prevalence odds ratios [Reference Rothman and Greenland14], initially only adjusted for year (termed ‘univariate’), then with multiple regression analyses including the key factors of interest (ethnicity, gestation, birth weight, NZDep2001 score), and factors that showed elevated risks in univariate analyses. A similar approach was adopted for the analyses of factors that affected length of hospital stay. Multicollinearity was tested for, and not found to be a significant problem in the multivariate model. Therefore, all variables were retained in the final analyses.
RESULTS
RSV was confirmed in 141 (61·3%) of 230 infants hospitalized with bronchiolitis. Of the 141 RSV-positive samples, 135 (95·7%) were typed as RSV subgroup A or B. One infant had RSV subgroup B detected during his first admission and subgroup A was identified 3 weeks later when he was readmitted with a new episode of bronchiolitis. No deaths were directly attributable to bronchiolitis. In 2003 and 2005, the epidemics were predominantly RSV subtype A (72·5% and 80·5%, respectively). By contrast, the 2004 epidemic was predominantly subtype B (60·0%).
Analysis of risk factors for RSV hospitalization and demographic variables
Table 1 shows the characteristics of the 141 RSV-positive infants, compared with all live hospital births during 2003–2005 in the Wellington region and the associated rate ratios (RRs) for RSV bronchiolitis hospitalization.
Table 1. Characteristics and multivariate analysis of 141 RSV-positive infants admitted with bronchiolitis during 2003–2005 to Wellington Hospital, compared with all live hospital births in the Wellington region during the same period
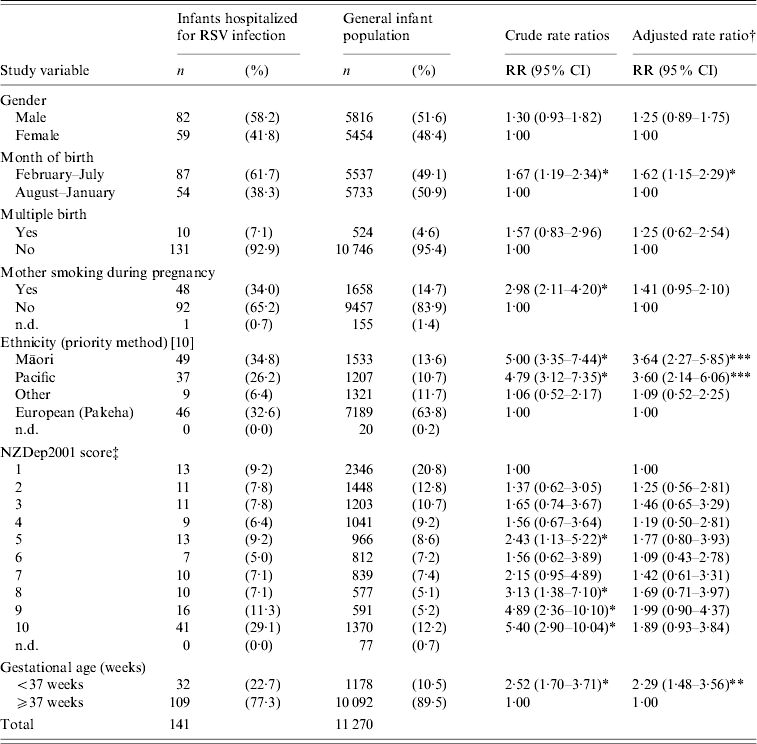
RSV, Respiratory syncytial virus; RR, rate ratio; CI, confidence interval; n.d., no data available.
† Multivariate rate ratio adjusted for all other variables in Table 1.
‡ Proxy measure for socioeconomic deprivation, where decile 1 represents the 10% least deprived and decile 10 the 10% most deprived of neighbourhoods.
* P⩽0·05, ** P⩽0·0005, *** P⩽0·0001.
Subjects were compared with patients hospitalized for bronchiolitis during 2003 and 2004 using the departmental database. The database for 2005 was incomplete at the time of writing. Overall, 66·5% of eligible patients (admitted during weekdays) were enrolled. The main reason for non-participation was discharge from hospital before research staff were able to approach their caregivers. This occurred predominantly when infants were admitted to hospital late in the week and were sent home over the weekend or early Monday morning. Database review showed non-participants (including those from weekends) were of similar age (91·0% aged <12 months at admission and 47·2% aged <6 months) to study subjects (87·9% aged <12 months, 55·3% aged <6 months). Ethnic distribution (38·2% European/other, 30·3% Māori, 31·5% Pacific) was also similar to study participants (Table 1).
The median age of infants hospitalized with RSV was 5·1 months (interquartile range 2·0–9·0). The mean and median length of hospitalization was 5·1 days [95% confidence interval (CI) 4·5–5·7] and 4·0 days (interquartile range 2·0–7·0), respectively. Fifteen (10·6%) RSV-positive infants had a major underlying illness (cardiac, respiratory, Down's syndrome or achondroplasia).
The prioritized ethnicity of RSV hospitalized infants varied significantly from the general infant population (Table 1) with Māori and Pacific children significantly over-represented in the hospitalized group. Multivariate analysis identified Māori or Pacific ethnicity as an independent risk factor for hospitalization. Infants who lived in more deprived areas (NZDep2001 deciles 8–10) were also significantly over-represented compared with the general infant population. Infants in more deprived areas comprised almost half (47·5%) of all study subjects. A total of 29% were in the lowest socioeconomic group and had a significant crude RR for hospitalization. However, when adjusted for other risk factors this was markedly reduced and no longer significant, indicating that the association of lower socioeconomic status with hospitalization was in part due to other factors controlled in the model (e.g. maternal smoking, gestational age and ethnicity).
One third (34·0%) of RSV-positive hospitalized infants had mothers who smoked during pregnancy, significantly more than the general infant population (14·7%). Although the crude RR showed maternal smoking during pregnancy to be significantly associated with hospitalization, this was no longer so in the multivariate analysis (Table 1). In this study, more than half (54·6%) of the RSV-positive hospitalized infants came from a household with one or more smokers. The most recent NZ data available regarding household tobacco smoke exposure is from the 1996 census, and indicate that just under one third of households with dependant children (<15 years) have one or more smokers [15].
The majority of RSV-positive infants (61·7%) were born in the 6 months preceding the RSV epidemic season (February–July, inclusive). Table 1 shows that infants born between February and July had a significantly increased risk for hospitalization, even when adjusted for other risk factors. Most infants had birth weights ⩾2500 g (79·4%), with 10·2% below the 10th gestation-adjusted weight percentile. Similarly, 10·3% had an admission weight below the 10th age-adjusted weight percentile (not shown in table). Of hospitalized RSV-positive infants, 85·8% had been breastfed and 45·4% were still breastfeeding (not shown in table).
Most RSV-positive infants came from a household in which other children lived; 67·9% shared a house with 1–2 other children, and 13·6% with ⩾3 other children (not shown in table). Most infants (63·5%) lived with two adults, and 8·5% lived with just one adult. More than half (57·6%) of RSV-positive infants in this study shared their sleeping space with one or more others. Only a small percentage (13·5%) attended day-care, which was expected since most RSV-positive infants were aged <6 months.
Analysis of risk factors for RSV disease severity
RSV disease severity was defined by the requirement of supplemental oxygen or assisted ventilation (Table 2) and by length of hospital stay (Table 3). Severe cases (n=34, 24%) were hospitalized for a mean of 8·8 days (95% CI 7·6–10·1), moderate (n=70, 50%) for 4·6 days (95% CI 4·0–5·2), and mild cases (n=37, 26%) for 2·6 days (95% CI 2·0–3·1). Infants aged <2 months at admission were more likely to have severe disease than moderate or mild disease; however, this was no longer significant in the multivariate analysis (Table 2). No significant correlations were identified between severe disease and: gestational age, ascribed ethnicity (Table 2), NZDep2001 score grouped by individual score or as less deprived (1–5) vs. more deprived (6–10), RSV subtype, household cigarette-smoke exposure, or sharing a sleeping space with others (not shown in table).
Table 2. Risk factors for severeFootnote † (compared with moderate/mild) RSV bronchiolitis in hospitalized children
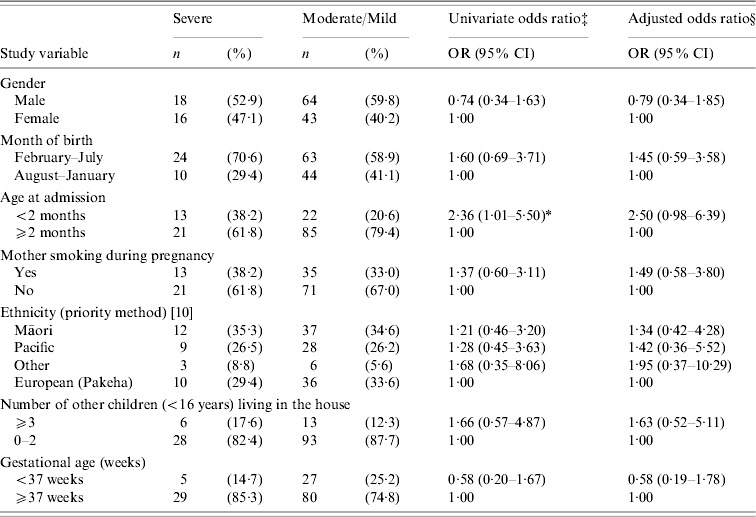
RSV, Respiratory syncytial virus; OR, odds ratio; CI, confidence interval.
† Severe=assisted ventilation or continuous positive airway pressure (CPAP); moderate=received supplemental oxygen; mild=no additional oxygen needed.
‡ Adjusted for year.
§ Adjusted for year and all other variables listed in the table.
* P⩽0·05.
Table 3. Risk factors for length of stay (⩾5 days vs. <5 daysFootnote †) in RSV-positive children hospitalized with bronchiolitis
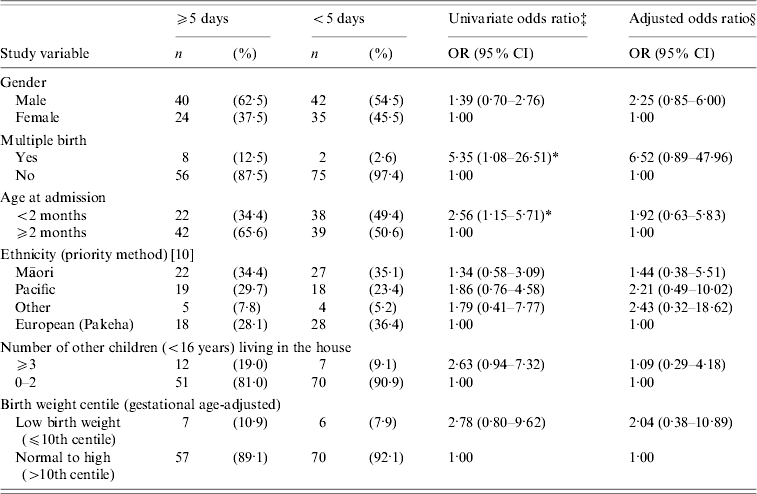
RSV, Respiratory syncytial virus; OR, odds ratio; CI, confidence interval.
† Mean length of hospitalization was 5·1 days (95% CI 4·5–5·7).
‡ Adjusted for year.
§ Adjusted for year and all other variables listed in the table.
* P⩽0·05
Age at admission of <2 months and multiple birth were both identified as risk factors for hospital stay of ⩾5 days; however, the risk for age at admission was no longer significant in the multivariate analysis (Table 3). While the odds ratio (OR) increased for multiple births in the multivariate analysis, it was no longer statistically significant (Table 3). Severe cases requiring CPAP or assisted ventilation were significantly more likely to stay in hospital for ⩾5 days (OR adjusted for year 35·65, 95% CI 8·01–158·62), illustrating the validity of length of hospital stay as an alternative measure of disease severity. Again, no significant associations were identified between hospital stay of ⩾5 days and: ascribed ethnicity, birth weight centile (Table 3), NZDep2001 score grouped by individual score or as less deprived (1–5) vs. more deprived (6–10), RSV subtype, gestational age, household cigarette-smoke exposure, or sharing a sleeping space with others (not shown in table).
DISCUSSION
This foremost study of RSV bronchiolitis in NZ identified three independent risk factors for hospitalization: birth between February and July, gestational age <37 weeks and Māori or Pacific ethnicity. The increased risk associated with pre-term delivery and birth within 6 months of the annual RSV season (presumably secondary to low protective maternal antibody titres) is similar to observations in the northern hemisphere [Reference Welliver6, Reference Holberg16]. Multiple regression analysis indicated the high rates in Māori and Pacific infants could only be partially accounted for by factors such as maternal smoking during pregnancy, the deprivation status of the area in which the infant lived, low birth weight, gender and month of birth.
Outside North America, few studies from developed countries have examined reasons for severe RSV bronchiolitis in indigenous and disadvantaged populations [Reference Smyth and Openshaw1, Reference Whitehall17]. However, in contrast to our findings, a recent Australian report concluded that the increased risk of hospitalization from RSV bronchiolitis in Indigenous infants was largely due to lower socioeconomic status-associated factors such as lower birth weight and maternal smoking [Reference Reeve18].
Native American and Alaskan infants also have higher hospitalization rates for RSV bronchiolitis than other populations [Reference Holman19]. Highest rates were found in remote rural regions of Alaska and the southwest, while urbanized Native Americans with a higher socioeconomic status had lower rates than the general US infant population. These rate differences were attributed to a combination of socioeconomic factors, such as household crowding and access to health care [Reference Holman19, Reference Bulkow20]. A recent retrospective Californian study also found that RSV hospitalization rates were higher among infants of lower socioeconomic status (defined by use of Medicaid), and in rural areas with high poverty rates [Reference Sangare, Curtis and Ahmad21]. Interestingly, Asian/Pacific infants and American Indian and Alaskan Native infants using Medicaid had lower hospitalization rates than non-hispanic white infants using Medicaid.
Our study had relatively limited information on factors such as maternal smoking and area-based deprivation, the latter being a crude measure of individual deprivation [Reference Salmond and Crampton22, Reference Salmond23]. In particular, it is probable that housing conditions and overcrowding play a role that is not fully captured by NZDep2001 scores. It is possible that RRs for Māori and Pacific infants might have reduced towards the null value of 1·0, had we been able to obtain more accurate information on these risk factors and control for them more fully.
Nevertheless, these analyses suggest there may be other untested factors, contributing to the higher incidence of RSV hospitalization in Māori and Pacific infants. Associations between severe RSV infection and polymorphisms of the surfactant protein gene loci [Reference Lofgren24], interleukin (IL)-4/IL-13 genes [Reference Puthothu25], the vitamin D receptor [Reference Janssen26] and many other gene loci [Reference Wilson27, Reference Amanatidou28] have been reported. However, no information currently exists on their prevalence in Māori and Pacific infants.
Furthermore, it is important to note that the ethnicity to which an individual ascribes encompasses their cultural and environmental practices as well as their genetic heritage [Reference Pearce29]. The contribution of each of these factors must be considered when seeking reasons behind the greater incidence of RSV hospitalization in Māori and Pacific infants.
As with Alaskan Native and remote rural Indigenous Australian infants [Reference Singleton30, Reference Chang31], Māori and Pacific children are also known to be at greater risk than NZ European/Pakeha children for other respiratory infections and pulmonary disease [Reference Grant32–Reference Twiss34]. High asthma hospitalization rates in Māori children were previously ascribed to genetic factors, but subsequent analyses found this was from disparities in access to primary health care rather than underlying disease susceptibility [Reference Ellison-Loschmann and Pearce35]. If hospital care was preferentially used by Māori or Pacific infants from lack of access to primary health care, or because of hospital physician practices, it might be expected that moderate or mild cases would be increased for Māori or Pacific infants. However, ethnicity was not associated with disease severity in this study, suggesting that these factors are unlikely to be important.
Our failure to identify risk factors for more severe RSV disease or hospitalization for ⩾5 days in the multivariate analysis are similar to an earlier review of NZ infants requiring assisted ventilation for bronchiolitis from all causes where only pre-term delivery was considered important [Reference Gavin, Anderson and Percival36]. This failure could be attributed to difficulties in detecting significant differences within a small and highly select population of infants with disease severe enough to warrant hospital admission. Instead, it might be more appropriate to compare RSV-infected infants managed solely in the community with those requiring hospitalization.
Our study reaffirms pre-term delivery and birth within 6 months of the RSV epidemic season as risk factors for hospitalization. It extends North American findings [Reference Reeve18, Reference Holman19] of increased risk for RSV bronchiolitis hospitalization among rural indigenous infants by finding a similar situation exists in a NZ urban environment. Factors contributing to this high risk for Māori and Pacific infants are probably a combination of host, environmental and behavioural factors that are worth investigating further, particularly given the possible long-term sequelae of severe RSV bronchiolitis [Reference Sigurs37–Reference Singleton39]. The information gained could also benefit ethnic minorities and indigenous children in other developed countries. Additional studies are planned to identify interventions that may reduce RSV hospitalization while awaiting safe and effective vaccines. This includes, for example, determining whether deficiencies in micronutrients that help regulate innate immunity are increased in Māori and Pacific populations resident in New Zealand.
ACKNOWLEDGEMENTS
We thank the staff of Ward 19 and the Diagnostic Laboratories at Wellington Hospital for their assistance in obtaining clinical samples. We thank Mr Keith Fisher, Capital and Coast District Health Board, for providing the dataset containing information of all live hospital births in the Wellington region 2003–2005. This study was funded by the New Zealand Lottery Health Grants Board, the Child Health Research Foundation of New Zealand, the Wellington Medical Research Foundation and the Rotary Club of Wellington Central. J. Kirman is a Sir Charles Hercus Health Research Fellow of the Health Research Council of New Zealand. The Centre for Public Health Research and the Research Centre for Māori Health and Development are supported by Programme Grants from the Health Research Council of New Zealand. C. Cohet was supported in part by a Massey University Postdoctoral Fellowship.
DECLARATION OF INTEREST
None.





