INTRODUCTION
Healthcare-associated infections (HAIs) cause significant morbidity and mortality in acute care settings [Reference Klevens1], partly due to increased antibiotic resistance in HAIs [Reference Marschall2]. Methicillin-resistant Staphylococcus aureus (MRSA) has been the focus of much research due to its major contribution to the morbidity and mortality of hospitalized patients [Reference Filice3–Reference Rubin6]. S. aureus can cause serious infections at many body sites and is one of the most common causes of bacteraemia [Reference Wisplinghoff7]. Approximately one-third of patients with bacteraemia caused by S. aureus develop local complications or distant septic metastases [Reference Fowler8]. These infections are even more complicated when the organism is resistant to methicillin or other anti-staphylococcal penicillins, and result in increased mortality, length of stay, and hospital costs [Reference Cosgrove9–Reference Shurland13].
Several researchers have attempted to identify predictors of MRSA bacteraemia in hospitals [Reference Blot12, Reference Libert14–Reference Bakowski20]. However, the majority of studies were limited by small samples, single-site settings and methodological issues such as inadequate control for severity of illness. Studies utilizing matching failed to employ statistical methods to adjust for lack of independence in cases and matched controls. Moreover, existing studies varied in the control group chosen; most studies used patients with methicillin-susceptible S. aureus (MSSA) bacteraemia as controls, which identified predictors of MRSA resistance in bacteraemia. However, researchers have hypothesized that using MSSA bacteraemia controls may overestimate the association between antibiotic use and MRSA bacteraemia since prior use of antibiotics such as oxacillin is likely to prevent infection with strains of bacteria that are susceptible to that particular antibiotic [Reference Harris21]. Other studies selected controls with no infection and identified predictors of bacteraemia due to S. aureus. However, many of these studies did not adequately control for the differences seen in severity of illness between the cases and controls, making it difficult to identify other pertinent risk factors. Additionally, most studies grouped community-associated infections and HAIs together, which may mask some important hospital-related risk factors.
The objective of this nested case-control study was to identify and compare risk factors for healthcare-associated MRSA bacteraemia using two sets of controls – controls with MSSA bacteraemia and non-infected controls – in a large sample of hospitalized patients.
METHODS
Data were obtained from a large healthcare system in New York City, compiled as part of a larger study aimed at assessing HAI costs (Distribution of the Costs of Antimicrobial Resistant Infections, 5R01NR10822). This system includes two tertiary academic health centres, a paediatric hospital, and a community hospital. As part of the system, these hospitals share one clinical data warehouse (CDW) which integrates data from over 20 clinical databases including laboratory, radiology, and diagnostic data sources among many others. As part of the larger study, data from the CDW was linked with routinely collected administrative and electronic health records.
Selection of cases and controls
We examined anonymized data on all admissions to the healthcare system from 2006 to 2008 (N = 3 19 945). We defined healthcare-associated bacteraemia as those that manifested at least 72 h after admission (3 days from admission). Cases and controls were defined using an electronic algorithm based on the Centers for Disease Control and Prevention's National Healthcare Safety Network (NHSN) definitions for primary bacteraemia [Reference Horan, Andrus and Dudeck22]. NHSN is a surveillance network to which hospitals report HAI rates, and NHSN definitions have become the standard for defining infections around the world [Reference Emori23]. For this study, NHSN definitions were modified to focus on electronically available data.
MRSA bacteraemia cases were defined according to the following criteria: (1) positive MRSA blood culture and, (2) no positive MRSA culture at other body sites within 14 days prior to positive blood culture. We used two sets of controls. MSSA bacteraemia controls were defined as those with: (1) positive MSSA blood culture and, (2) no positive MSSA at other body sites within 14 days prior to positive blood culture. Non-infected controls were defined as those with no positive blood culture for any organism. MRSA bacteraemia cases were compared to MSSA bacteraemia controls to determine the risk factors for methicillin resistance (unmatched). Additionally, cases were matched to non-infected controls on age ( ± 5 years), minimum length of exposure (number of days hospitalized prior to development of bacteraemia in cases), early intensive-care unit (ICU) stay (defined as admission to an ICU in the first 3 days of hospital stay) and hospital to determine risk factors for MRSA bacteraemia (using 2:1 matching).
Data elements
The demographic factors examined were gender and age at discharge. To investigate the role that prior hospitalization plays in increasing risk for bacteraemia, we examined history of hospitalization in the four study hospitals and days since the hospitalization in the prior year, as well as length of stay during last hospitalization. History of stay at a skilled nursing facility (SNF) within the prior year was defined based on the admission source from administrative data and by matching admission addresses to known SNF.
Data on the following clinical risk factors were also examined using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes and the following indicators present on admission: diabetes, malignancy, trauma, open wound, chronic dermatitis, renal failure, burns (any or third degree), history of major organ transplant, substance abuse history, asthma, chemotherapy, congestive heart failure, cirrhosis, chronic obstructive pulmonary disease, cardiovascular disease, decubitus ulcer, hepatitis B and C infection, HIV infection, neurological disease, rheumatoid arthritis and tracheostomy. A Charlson co-morbidity score was calculated as a measure of the patient's health status at admission [Reference Charlson24].
Antibiotic exposure was defined as overall exposure to an antibiotic during the period at risk (defined as having received at least one dose of the specific antibiotic in the period at risk), and exposure to specific classes of antibiotics (i.e. aminoglycosides, carbapenems, cephalosporins, glycylcylines, macrolides, monobactams, penicillins, polypeptides, quinolones, sulfonamides, tetracyclines and other). Immunosuppressive medication use was also examined as a risk factor.
Use of central venous and urinary catheters prior to infection was investigated as a dichotomous and a continuous variable (total days of use). The occurrence of the following procedures during the current hospitalization were assessed: specialized cardiac procedure (i.e. cardiac catheterization or angiography, coronary angioplasty, vascular stenting), intubation, dialysis, feeding tube insertion, major organ transplant, general anaesthesia, open biopsy, any operating-room procedure performed in the hospitalization lasting ⩾30 min, and major operating-room diagnostic or therapeutic procedure defined according to the Healthcare Cost and Utilization Project (HCUP) classifications [25].
For the comparison of cases with MSSA bacteraemia controls, exposure was defined as the period before the development of bacteraemia in both cases and controls. For the comparison of cases with matched non-infected controls, exposure was defined as the number of hospital days prior to infection for each index case and the corresponding period at risk for the matched control (i.e. occurring within the same number of hospital days as for the matched index case).
Statistical analysis
Data were analysed in Stata v. 11.1 (Stata Corporation, USA). In the first analysis, we assessed risk factors for MRSA resistance in bacteraemia by comparing MRSA bacteraemia cases to MSSA bacteraemia controls. Mann–Whitney tests for continuous non-parametric variables and χ 2 tests or Fisher's exact tests for categorical variables were used in bivariate analysis. Multivariable logistic regressions were used to assess the independent effect of these variables on the risk of developing a resistant bacteraemia. The second analysis assessed risk factors for MRSA bacteraemia by comparing cases with MRSA bacteraemia and non-infected matched controls using conditional logistic regression to account for matching on age, period at risk, early ICU stay and hospital.
For both analyses, variables with P ⩽ 0·1 in bivariate analysis were included in multivariable analysis to estimate the probability of MRSA bacteraemia. Potential confounders were added one by one into the model, and if coefficient estimates of a covariate changed by >10%, the variable was considered a confounder and added to the model. Effect modification between covariates was evaluated by testing interaction terms for variables that were conceptually potential effect modifiers. Multi-collinearity was assessed by examining tolerance and variance inflation factors and goodness of fit was assessed using the Hosmer–Lemeshow goodness-of-fit test and Akaike's Information Criterion.
RESULTS
Comparison of MRSA bacteraemia cases and MSSA bacteraemia controls
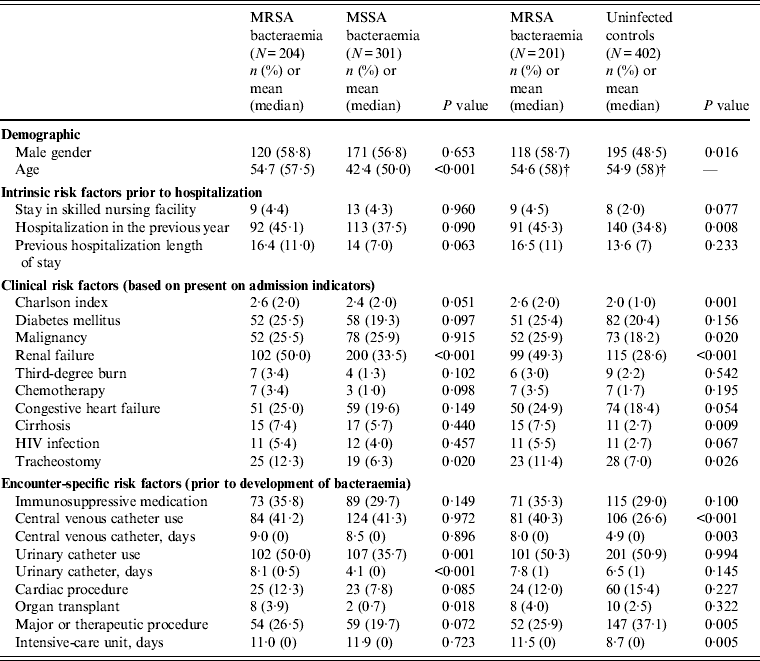
A total of 204 MRSA bacteraemia cases and 301 MSSA bacteraemia controls were identified during the study period. The number of hospital days prior to the development of bacteraemia did not differ significantly between the cases and controls (mean = 23·9 ± 36·3 and 21·7 ± 28·4, respectively, P = 0·145). Significant demographic, clinical and encounter-based predictors of MRSA bacteraemia in bivariate analysis are summarized in Table 1 (full results shown in the Supplementary online Appendix). Cases were more likely than controls to be older (P < 0·001), have renal failure (P < 0·001) and a tracheostomy (P = 0·02) present on admission, as well as having a urinary catheter (P = 0·001), dialysis (P = 0·009) and major organ transplant (P = 0·018) during their encounter prior to the development of bacteraemia. Additionally, cases had higher Charlson severity-of-illness scores than MSSA bacteraemia controls (P = 0·051). In bivariate analysis, quinolone use was the only antibiotic significantly associated with MRSA bacteraemia (P = 0·001); overall antibiotic and monobactam use approached statistical significance (P values of 0·059 and 0·056, respectively).
Table 1. Bivariate comparison of characteristics of MRSA bacteraemia cases with MSSA bacteraemia and uninfected controls*

* For matched analysis, defined exposure for controls within period at risk for matched case.
† Used in matching MRSA bacteraemia cases to non-infected controls.
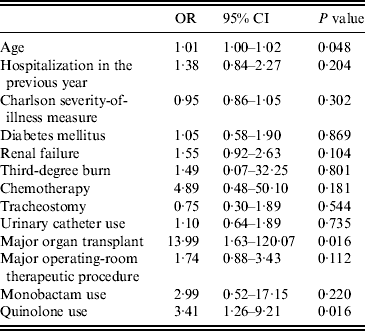
Table 2 shows results of the multivariable model. The three independent risk factors for MRSA bacteraemia were older age (OR 1·01, 95% CI 1·00–1·02), major organ transplant during current hospitalization (OR 14·0, 95% CI 1·63–120·07) and quinolone use (OR 3·41, 95% CI 1·26–9·21). No differences in the models were seen whether urinary catheter exposure was assessed as a dichotomous variable or as the number of catheter days prior to bacteraemia (data not shown).
Table 2. Multivariable analysis of risk factors for MRSA bacteraemia using MSSA bacteraemia controls including antibiotic use (N = 330)

OR, Odds ratio; CI, confidence interval.
Comparison of MRSA bacteraemia cases and non-infected matched controls
Overall, 1:2 matching on early ICU stay, age, hospital and minimum time at risk was successful for 201/204 MRSA bacteraemia cases. Table 1 shows the bivariate comparison of MRSA bacteraemia cases and matched non-infected controls. Cases and controls differed significantly on gender (P = 0·016), hospitalization in the previous year (P = 0·008), severity of illness (P = 0·001), history of malignancy (P = 0·020), renal failure (P < 0·001), cirrhosis (P = 0·009), tracheostomy (P = 0·026), central venous catheter use (P < 0·001), major operating-room therapeutic procedure (P = 0·005) and days spent in an ICU (P = 0·005). As in the comparison of cases with MSSA bacteraemia controls, cases were significantly more likely to have been exposed to quinolones in the period at risk than their corresponding non-infected controls (OR 4·2, P = 0·003).
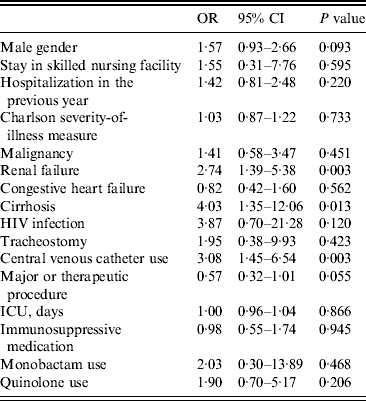
In the multivariable model (Table 3), cases were more likely than controls to have renal failure (OR 2·74, 95% CI 1·39–5·38), cirrhosis (OR 4·03, 95% CI 1·35–12·06), and a central venous catheter (OR 3·08, 95% CI 1·45–6·54). After controlling for the other risk factors, quinolone exposure was no longer a significant predictor of MRSA bacteraemia (OR 1·90, 95% CI 0·7–5·17). As in the previous model, controlling for other risk factors, cases were less likely than controls to have a major operating-room therapeutic procedure during the time at risk although the association was not statistically significant (OR 0·57, 95% CI 0·32–1·01). Central venous catheter use had the same impact on risk of bacteraemia whether it was assessed as a continuous or dichotomous variable (data not shown).
Table 3. Multivariable analysis of risk factors for MRSA bacteraemia using non-infected controls including antibiotic use (N = 358)

OR, Odds ratio; CI, confidence interval.
DISCUSSION
We performed a large case-control study to evaluate risk factors for MRSA bacteraemia. Although we improved upon previous studies by employing a large sample, using two control groups, and matching to adjust for underlying differences between cases and uninfected controls, we found similar results to those published previously. We found that risk factors for MRSA bacteraemia differed depending on the control group chosen. This is in contrast to a study assessing risk factors for MRSA surgical site infections (SSIs) which also used two sets of controls; 84 MRSA SSI patients were compared to 64 MSSA SSI patients and 167 patients without SSI, potentially allowing for the differentiation between risk factors for MRSA SSI and SSI due to any S. aureus [Reference Chen26]. The researchers showed that requiring assistance in three or more activities of daily living, and wound class were independently associated with MRSA SSI using both controls groups.
A study by Graffunder & Venezia of 121 MRSA patients and 123 MSSA controls, identified levofloxacin, belonging to the quinolone class, and macrolides as independent risk factors for MRSA infection (although not specifically bacteraemia) [Reference Graffunder and Venezia27]. We also identified macrolides as risk factors in bivariate analysis but macrolide use failed to remain an independent predictor of MRSA bacteraemia when entered into a multivariable model. Importantly, in our study, quinolone use was an independent predictor of MRSA bacteraemia compared to MSSA bacteraemia but not in the comparison of MRSA bacteraemia patients and non-infected controls. This is consistent with the results obtained by Ernst and colleagues who evaluated the importance of control group selection in studies assessing the association between use of antibiotics and MRSA bacteraemia and utilized two sets of controls [Reference Ernst18]. Specifically, these researchers argued that the appropriate control group when assessing antibiotic exposure as a potential risk factor in a case-control study is a non-infected control group, since those patients who received antibiotics effective in the treatment of MSSA would be much less likely to develop an infection with MSSA. Therefore, patients with antibiotic exposure may be less likely to be selected as controls in the case-control study, leading to selection bias and an overestimation of the effect that antibiotic exposure has on the development of MRSA bacteraemia. Indeed, as in our study, these researchers observed a significant association between exposure to antibiotics and infections with MRSA bacteraemia when compared to MSSA bacteraemia controls but not when the non-infected control group was utilized. One of the weaknesses of this study, in addition to a small sample size, was the fact that the researchers matched cases and controls but did not utilize statistical methods appropriate for matched data. Despite this limitation, the results of the Ernst et al. study are confirmed by our findings, which underscore the importance of choosing appropriate controls depending on the risk factors under examination in case-control studies. Researchers must carefully consider their choice for a control group in light of the specific research question under investigation and must be aware of the potential limitations of choosing a particular control group in terms of the research question being asked [Reference Harris21, Reference Harris28].
In a study of 60 MRSA bacteraemia patients and 240 non-infected controls, Bakowski et al. identified severity of illness and use of central venous catheters as independent risk factors for MRSA bacteraemia [Reference Bakowski20]. In addition, previous surgery was protective against acquiring MRSA bacteraemia. The researchers chose an uninfected control group instead of MSSA bacteraemia controls because they aimed to identify risk factors for MRSA bacteraemia and not risk factors for methicillin resistance in bacteraemia. However, large differences in disease severity between the cases and controls may have masked other risk factors for infection. Our study identified similar results in that the comparison of MRSA bacteraemia with non-infected controls identified central venous catheter use as the only independent encounter-based risk factor for MRSA bacteraemia and identified ‘major operating-room therapeutic procedure’ as a protective factor, after controlling for other demographic and clinical risk factors. Even after matching cases and controls on age, early ICU stay and minimum time at risk, important differences in underlying severity of illness seem to be present as evidenced by ‘major operating-room therapeutic procedure’ as protective in terms of developing infection. A potential explanation is that patients admitted specifically to undergo a procedure may be healthier than those admitted for another reason and therefore may be less likely to develop MRSA bacteraemia. This finding underscores the need for carefully chosen comparison groups and the importance of careful consideration of the underlying differences in severity of illness between comparison groups, perhaps necessitating the use of more stringent matching procedures, e.g. reason for admission.
Our study identified solid organ transplant as a risk factor for MRSA bacteraemia using the MSSA bacteraemia control group but not the non-infected control group. One potential explanation for this association may be that patients who undergo solid organ transplant may be more likely to have other comorbid conditions such as diabetes mellitus or more likely to undergo treatments such as haemodialysis that puts them at increased risk of acquiring a resistant infection. Another potential explanation may be that patients who receive transplants are likely to be hospitalized for a longer period of time compared to patients who do not which in turn increases their risk of an antibiotic resistant infection.
One study limitation was dependence upon data available in electronic medical records. Numerous studies have shown that patients colonized with S. aureus are at increased risk of infection, underscoring the importance of S. aureus carriage as an endogenous source of infection [Reference Tacconelli29–Reference Bert31]. However, since this study was retrospective, data on certain potential risk factors such as previous colonization were not available. Moreover, in order to utilize a dataset of this magnitude, it was necessary to modify NHSN definitions to focus on electronically available data. Thus it is possible that secondary bacteraemia were mistakenly misclassified as primary bacteraemia and vice versa since only microbiological data was used to determine whether an infection existed at another site. Another limitation is lack of complete data on antibiotic use in two of the four hospital sites for part of the study period. Furthermore, although this is a large study focusing on MRSA bacteraemia risk factors, it was limited to hospitals in New York city, which may limit generalizability of the results.
A major strength of this analysis was the large sample size, which gives sufficient power to identify pertinent risk factors. Since this study included all cases of MRSA and MSSA bacteraemia in a 3-year period it should not be subject to selection bias. Data were obtained from four hospitals which served different patient populations, increasing the generalizability of the results. In addition, the use of two control groups allowed for the identification and comparison of risk factors for MRSA bacteraemia and resistance in bacteraemia.
We performed a case-control study to assess risk factors for MRSA bacteraemia using two sets of controls; risk factors for MRSA bacteraemia differed greatly depending on the control group. Our results emphasize the need for careful selection of appropriate control groups in case-control studies depending on the specific research question being investigated, especially when studying antibiotics as potential risk factors for MRSA bacteraemia, as well as the need to carefully adjust for underlying severity of illness. Further research is needed to identify proper controls in these types of studies. Moreover, additional research to uncover the inter-relationships between different risk factors for MRSA bacteraemia would aid in our understanding of the biological mechanisms through which these infections are acquired.
SUPPLEMENTARY MATERIAL
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/S0950268813000174.
ACKNOWLEDGEMENTS
The project described was supported by Award Number 5R01NR10822 from the National Institutes of Nursing Research.
DECLARATION OF INTEREST
None.