Introduction
Primary care clinicians play a principal role in providing front-line care to patients presenting with influenza-like illness (ILI) during influenza pandemics [Reference Kunin1]. This includes prioritising influenza vaccination in high-risk groups [2, 3] and accurately targeting antibiotics and antiviral medications to maximise clinical benefit without driving antimicrobial resistance [Reference Goossens4, Reference Stephenson5].
The highest rates of primary care consultations for influenza A-attributable respiratory disease in the UK are observed in children under 5 years of age, while the highest seasonal incidence rates for influenza B occur in children aged 5–17 years [Reference Fleming6]. Universal childhood vaccination strategies for seasonal influenza are already being implemented in the USA [3] and UK [7].
Preliminary data from England suggest that vaccination of children aged 4–11 years may have a direct impact on reducing illness absenteeism in primary schools [Reference Green8]. However, no significant indirect impact on illness absenteeism in secondary schools has been demonstrated [Reference Green8]. There is also still insufficient evidence on whether universal childhood vaccination is effective at reducing influenza-related complications and hospitalisations, and which types of communities are likely to benefit most from this type of approach [Reference Yin9].
Targeted strategies which prioritise groups at highest risk of clinical deterioration are therefore still important in primary care, particularly during influenza pandemics, when there is increased demand on health care resources and suitable vaccines may not initially be available [Reference Lim10]. A systematic review of studies involving children with seasonal or pandemic influenza/ILI identified neurological conditions, premature birth, sickle cell disease, immunosuppression, diabetes mellitus and age under 2 years as risk factors for hospitalisation [Reference Gill11]. However, these findings are mainly based on data from studies conducted in hospital ambulatory care settings, and represent risk factors for all-cause hospitalisation rather than influenza-related complications. Additionally, current definitions of high-risk groups do not provide evidence-based guidance on which risk factors are particularly relevant to paediatric primary care populations [2, 3].
The present study therefore aims to identify risk factors for influenza-related complications in children presenting in primary care using routinely collected linked data from the Clinical Practice Research Datalink (CPRD).
Methods
Source data and population
CPRD (http://www.cprd.com) provides anonymised routinely collected data from patients presenting in UK primary care [Reference Herrett12]. Linkage to Hospital Episode Statistics (HES), Office for National Statistics mortality data and Index of Multiple Deprivation (IMD) scores are available for a subset of CPRD practices in England, representing about 58% of patients registered at practices contributing to CPRD. The HES database contains details of admissions to NHS hospitals and NHS-funded admissions to private or charitable hospitals in England [Reference Herbert13].
We extracted data from the CPRD records of children aged 17 years or younger who consulted with influenza/ILI during the 2009/10 influenza pandemic (i.e. between 27 April 2009 and 23 May 2010). We excluded records that did not meet CPRD quality standards [Reference Herrett12] and where data were not available for at least 12 months before the index consultation in children aged 1 year or older, or at least 30 days before the index consultation in children younger than 12 months of age.
Potential risk factors
Potential risk factors were defined as binary variables based on the records of pre-specified Read codes for neurological, haematological, metabolic, cardiac, renal, liver and respiratory conditions, as well as premature birth and non-haematological malignancies. Supplement S1.1 describes these definitions in further detail.
Outcomes
Our primary outcome was influenza-related complications recorded within 30 days of presentation with influenza/ILI. These included respiratory, cardiac, neurological and renal complications [Reference Meier14]. Secondary outcomes (all within 30 days of presentation with influenza/ILI) were pneumonia, influenza-related complications requiring further intervention (prescription of medication, further investigations or hospitalisation), hospitalisation or death due to influenza-related complications and all-cause hospitalisation or death. At the request of journal reviewers, ‘pneumonia or hospitalisation’ was included as an additional secondary outcome. Supplement S1.2 provides full details of how we defined and obtained data for these outcomes.
Potential confounders
Potential confounders considered in this study were: age, sex, socio-economic deprivation, vaccination status (2008/9 seasonal influenza, pandemic influenza, pneumococcal conjugate vaccine and Haemophilus influenzae b), prescription of other medications at the index presentation with influenza/ILI (e.g. corticosteroids, antibiotics, antivirals), presence of other potential risk factors and acute hospitalisations during the 12-month period before the index presentation. Socio-economic deprivation was measured based on IMD score quintiles at the Office for National Statistics small area level (100 houses) using the patient's postcode. For children aged <12 months at the index presentation, baseline data on acute hospitalisations between the date of birth and the date of the index presentation were extracted.
Data analysis
Baseline data on potential risk factors and confounders were summarised using numbers and percentages for categorical variables and means and standard deviations for continuous variables. To minimise the possibility of unintentional disclosure, categorical variables with fewer than five patient records were either not reported or combined with related variables. Data on duration between the index consultation and influenza-related complications were summarised as medians and interquartile ranges.
Statistical analyses were conducted using Stata version 14. To examine the association between potential risk factors and each of our outcomes, we performed logistic regression to calculate odds ratios (ORs) with 95% confidence intervals (CIs) for each potential risk factor, both unadjusted and adjusted for potential confounders. Age was modelled as a continuous fractional polynomial (Stata command mfp). We created a ‘missing’ category for our variable on socio-economic deprivation to use in our main analysis where IMD score data were not available. The outcomes all-cause hospitalisation and hospitalisation due to influenza-related complications were analysed using only CPRD records which were linkable to HES data.
Subgroup analyses were conducted according to three age categories (0–4, 5–11, 12–17 years). Pre-specified sensitivity analyses were undertaken in children whose CPRD records were linked to both IMD score and inpatient HES data, and to examine asthma requiring treatment with inhaled corticosteroids or other preventer therapies as a potential risk factor [15].
The project was approved by the CPRD Independent Scientific Advisory Committee (protocol number 15_252R). The protocol was made available to the journal reviewers.
Results
Study population
Our study population included 16 779 children who presented with ILI at CPRD general practices during the 2009/10 influenza pandemic. Table 1 summarises the baseline characteristics of these children. Pandemic influenza vaccination was only recorded in 100 children (0.6%) and the 2008/9 seasonal influenza vaccination in 715 children (4.3%). Antivirals were prescribed at the index consultation in 4037 children (24.1%) and antibiotics in 985 children (5.9%).
Table 1. Baseline characteristics of study population (N = 16 779)
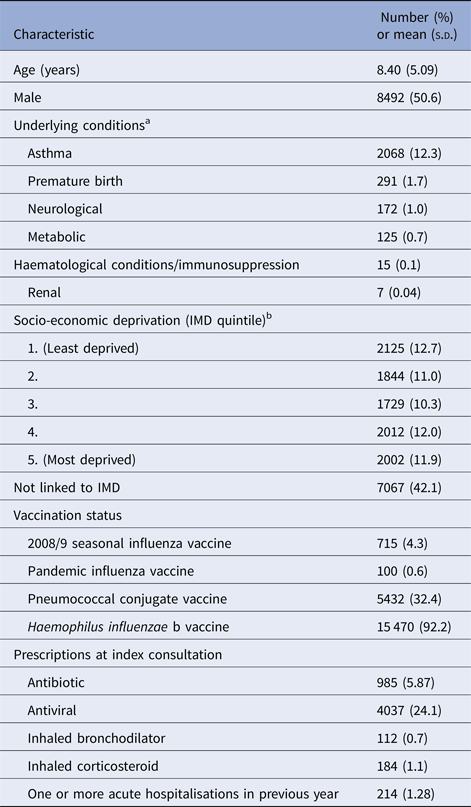
s.d., standard deviation; IMD, Index of Multiple Deprivation.
a One or more underlying conditions recorded in 2575 children.
b IMD quintiles based on IMD scores according to patient's postcode at the Office for National Statistics small area level (100 houses). Linked IMD score data available for 9712 children.
At least one underlying condition was present in 2575 children (15.4%). Asthma was the most prevalent condition (n = 2068, 12.3%). Neurological conditions were coded in 172 children (1.0%) of whom 146 had epilepsy. Metabolic conditions were found in 125 children (0.7%) of whom 95 had diabetes mellitus. Haematological or immunological conditions were only recorded in 15 children, renal conditions in seven children and non-haematological malignancies in fewer than five children. No children were recorded as having cardiac or liver conditions.
Influenza-related complications were recorded in 1339 of 16 779 children (8.0%). Median time to development of an influenza-related complication following the index consultation was 1 day (interquartile range 0–8 days). Forty-six per cent of complications (617/1339) were observed on the same day as the index consultation. Complications requiring intervention were observed in 668 children (4.0%) and pneumonia in 207 children (1.2%). Influenza-related complications were recorded in 695 of 5503 children aged 0–4 years inclusive (13%), accounting for just over half the total number of children who developed influenza-related complications (695/1339, 52%).
All-cause hospitalisations occurred in 116 of 9717 children whose CPRD records were linked to HES data (1.2%). Around half of all-cause hospitalisations (55/116, 47.4%) and hospitalisations due to influenza-related complications (32/57, 56.1%) were observed in children aged 0–4 years inclusive.
Risk factors for influenza-related complications
Table 2 summarises crude and adjusted ORs with 95% CIs in relation to influenza-related complications, complications requiring further intervention or pneumonia. Univariable analyses did not identify any statistically significant risk factors for these outcomes. However, after adjustment for baseline covariates and other risk factors, asthma was found to be a statistically significant risk factor for influenza-related complications (adjusted OR 1.48, 95% CI 1.21–1.80, P < 0.001), complications requiring intervention (adjusted OR 1.44, 95% CI 1.11–1.88, P = 0.007) and pneumonia (adjusted OR 1.64, 95% CI 1.07–2.51, P = 0.024). The association between neurological conditions and influenza-related complications requiring intervention was of borderline statistical significance (adjusted OR 1.94, 95% CI 1.01–3.75, P = 0.047).
Table 2. Crude and adjusted odds ratios with 95% confidence intervals in relation to influenza-related complications (N = 16 779)

CI, confidence interval; n, number of children; N, number of children with outcome event.
a Adjusted for age, sex, socio-economic deprivation, vaccination status (2008/9 seasonal influenza vaccine, pandemic influenza vaccine, pneumococcal conjugate vaccine and Haemophilus influenzae b), prescription of medications at index consultation (antivirals, antibiotics, antivirals×antibiotics, inhaled bronchodilators, inhaled corticosteroids), presence of other underlying conditions and acute hospitalisation in the previous year.
In the multivariable model, prescription of antibiotics or antiviral medications at the index consultation was associated with significantly reduced likelihood of influenza-related complications (antibiotics: adjusted OR 0.38, 95% CI 0.26–0.55, P < 0.001; antivirals: adjusted OR 0.33, 95% CI 0.28–0.4, P < 0.001). In contrast, concurrent prescription of both antibiotics and antivirals together was associated with significantly greater likelihood of influenza-related complications (adjusted OR 4.06, 95% CI 2.02–8.13, P < 0.001). Since this interaction was statistically significant, an interaction term for prescription of antibiotics and antivirals was also included in the multivariable model.
Risk factors for hospitalisation
CPRD records of 9717 of the 16 779 included children (58%) were eligible for linkage to HES data. All-cause hospitalisations were recorded in 116 children (1.2%). Following the index consultation, median time to hospital admission was 2 days (interquartile range 0–16 days). Thirty-five children were admitted to hospital on the same day as the index consultation (30.2%). Nearly half of hospitalisations were coded as being for influenza-related complications (n = 57, 49.1%). Median time to hospitalisation for influenza-related complications was 1 day (interquartile range 0–12 days). Sixteen children were admitted to hospital on the same day as the index consultation (28.1%). Pneumonia or hospitalisation was recorded in 224 children (2.3%). No deaths were recorded in our study population.
Table 3 summarises crude and adjusted ORs with 95% CIs in relation to all-cause hospitalisations and hospitalisations due to influenza-related complications. The presence of neurological conditions was found to be a statistically significant risk factor for all-cause hospitalisation in both crude and adjusted analyses (crude OR 3.57, 95% CI 1.29–9.89, P = 0.014; adjusted OR 4.25, 95% CI 1.49–12.06, P = 0.007). Neurological conditions were also associated with significantly greater risk of pneumonia or hospitalisation (crude OR 3.30, 95% CI 1.51–7.19, P = 0.003; adjusted OR 3.62, 95% CI 1.62–8.08, P = 0.002).
Table 3. Crude and adjusted odds ratios with 95% confidence intervals in relation to hospitalisation (n = 9717)

CI, confidence interval; n, number of children; N, number of children with outcome event; NC, not calculable.
a Adjusted for age, sex, socio-economic deprivation, vaccination status (2008/9 seasonal influenza vaccine, pandemic influenza vaccine, pneumococcal conjugate vaccine and Haemophilus influenzae b), prescription of medications at index consultation (antivirals, antibiotics, antivirals×antibiotics, inhaled bronchodilators, inhaled corticosteroids), presence of other underlying conditions and acute hospitalisation in the previous year.
Asthma was a statistically significant risk factor for hospitalisation due to influenza-related complications after adjustment (adjusted OR 2.45, 95% CI 1.08–5.55, P = 0.031), but was not a risk factor for all-cause hospitalisation (crude OR 1.10, 95% CI 0.63–1.93, P = 0.740; adjusted OR 1.53, 95% CI 0.81–2.86, P = 0.188) or for pneumonia or hospitalisation (crude OR 1.01, 95% CI 0.66–1.53, P = 0.979; adjusted OR 1.28, 95% CI 0.8–2.05, P = 0.300).
In the multivariable model, prescription of antiviral medications was associated with a significantly reduced likelihood of influenza-related hospitalisations (adjusted OR 0.43, 95% CI 0.19–0.94; P = 0.036), all-cause hospitalisations (adjusted OR 0.60, 95% CI 0.36–0.99; P = 0.044) and pneumonia or hospitalisation (adjusted OR 0.3, 95% CI 0.19–0.47, P < 0.001).
Subgroup and sensitivity analyses
After adjustment for baseline covariates and other risk factors, asthma was a significant risk factor for pneumonia and hospitalisations due to influenza-related complications in children aged 4 years and younger (Supplement S2). Asthma was also a significant risk factor for influenza-related complications in children aged 5–11 years and 12–17 years after adjustment. The presence of a neurological condition was a risk factor for all-cause hospitalisation in children aged 5–11 years.
Supplement S3 summarises the results of our pre-specified sensitivity analyses on asthma requiring preventer therapy (Supplement S3.1) and CPRD records linked to both IMD score and inpatient HES data (Supplement S3.2). The findings of these analyses were consistent with those of our main analyses. We also conducted post hoc sensitivity analysis excluding children in whom influenza-related complications were recorded on the same day as the index consultation. The findings of this analysis were broadly consistent with the main analysis. However, asthma was no longer a significant risk factor for pneumonia (adjusted OR 0.99, 95% CI 0.3–3.29, P = 0.98). The association between antibiotic prescriptions and influenza-related complications was also no longer statistically significant (adjusted OR 0.74, 95% CI 0.51–1.08, P = 0.115). There were insufficient data to estimate an OR for neurological conditions in relation to complications requiring intervention.
Discussion
Principal findings
Our study provides a comprehensive assessment of risk factors for influenza-related complications and all-cause hospitalisations in children using routinely collected data from a large UK primary care cohort. Asthma is a strong risk factor for influenza-related complications, including pneumonia, in children presenting with influenza/ILI in primary care. Children with neurological conditions, mainly epilepsy (which is not mentioned in current risk group definitions), are at increased risk of all-cause hospitalisation, but not influenza-related complications. At least half of influenza-related complications and hospitalisations occur within 1 day of initial presentation in primary care.
Comparison with existing literature
The rapid onset of influenza-related complications which we observed in our cohort is consistent with previous reports that around 80% of intensive care admissions among children with laboratory-confirmed influenza occur within 24 h of hospitalisation [Reference Li-Kim-Moy16].
Previous systematic reviews have identified asthma as a risk factor for pneumonia [Reference Mertz17] and found that neurological conditions are associated with greater risk of all-cause hospitalisation [Reference Gill11, Reference Mertz17]. However, these analyses were based on data from adults and children [Reference Mertz17] and did not adjust for important potential confounders, including socio-economic deprivation and vaccination status [Reference Gill11, Reference Mertz17].
Neuromuscular and neurocognitive disorders have previously been identified as risk factors for pneumonia in patients with seasonal influenza [Reference Mertz17]. However, we did not observe a significant association between neurological conditions and influenza-related complications or hospitalisations. This may have been due to health care professionals using different codes to record complications, or having a lower threshold for admitting these children to hospital for observation, since clinical prognosis is reported to be worse if complications do develop [Reference Millman18].
We did not find premature birth or diabetes to be significant risk factors in our cohort, although these have previously been reported as risk factors in children presenting in hospital ambulatory care settings [Reference Gill11]. This may reflect differences between these settings in the complexity and severity of these conditions among children presenting with influenza/ILI. Coding of premature birth may also be less reliable in primary care records. Additionally, we did not have sufficient data to examine whether immunosuppression was a risk factor in primary care as well as hospital ambulatory care settings [Reference Gill11] due to the limited number of children with haematological or immunological conditions in our cohort. This may reflect recommendations for these children to be referred early or seen directly in hospital when acutely unwell to facilitate prompt management of suspected neutropenic sepsis [19].
Our observation that children who were prescribed antibiotics or antivirals were less likely to develop influenza-related complications should be interpreted with caution, since it was not possible to adjust for severity of the acute illness episode or other unmeasured confounders. The statistically significant interaction we observed between antibiotic and antiviral prescriptions may suggest some confounding by illness severity. Lower re-attendances with cough within 1 month have also been observed in patients with acute lower respiratory tract infections given immediate or delayed prescriptions for antibiotics [Reference Little20].
Strengths and limitations
Our study identifies risk factors for influenza-related complications of direct relevance to children presenting in primary care, where most influenza/ILI episodes are initially assessed. Linkage to inpatient HES data and IMD score data enabled more detailed analyses than have previously been possible on unlinked routinely collected primary care data from the General Practice Research Database [Reference Meier14].
Our methods of identifying relevant consultations using consultation codes for ILI are likely to be robust, given that these codes were used more frequently during the 2009/10 pandemic, with the highest peak in consultations observed in children under 15 years of age [Reference Hardelid21]. Studying consultations during the 2009/10 pandemic also helped enrich our sample for patients with influenza and minimise potential confounding due to antiviral treatment at the index consultation, since during the pandemic, antivirals were recommended in all patients with influenza/ILI, not just those considered to be at greater risk of complications.
The available data allowed us to identify risk factors for influenza-related complications managed in the community, and to examine risk factors for hospitalisations due to influenza-related complications separately from all-cause hospitalisations. We also adjusted our analyses for a range of potential confounders, including socio-economic status, vaccination status and prescription of medications at the index consultation.
To increase our focus on risk factors for hospital admissions for clinical deterioration, we defined hospitalisation outcomes as hospital admissions lasting 24 h or longer. Previous studies did not specify the minimum duration of hospital admissions which they considered as hospitalisation outcome events [Reference Gill11, Reference Mertz17] and may therefore have included a considerable proportion of admissions for short periods of observation rather than treatment of complications. In our cohort, nearly half of hospitalisations coded in HES were for <24 h (110/226, 48.7%).
Our main limitation was the lack of an established linkage between data from the National Pandemic Flu Service (NPFS) and CPRD. This linkage would have been highly informative, as during the 2009/10 influenza pandemic, almost six times as many patients contacted the NPFS instead of their general practice for advice on influenza/ILI [Reference Rutter22]. Additionally, CPRD records may not have contained complete data on antiviral medications dispensed during the 2009/10 pandemic, since general practices did not consistently record allocation of ‘flu vouchers’, which were required to authorise supply of antivirals from the national stockpile.
We did not have sufficient data to assess haematological or immunological conditions, renal conditions, non-haematological malignancies, cardiac conditions or liver conditions, which may also be important risk factors for influenza-related complications in children. Our definition of influenza-related complications was based on the definition used in a previously published analysis of data from the General Practice Research Database [Reference Meier14]. This definition was intentionally broad to facilitate inclusion of the wide range of complications managed in the community, as well as allow for variations in coding practices among primary care clinicians. However, we recognise that certain consultation codes may be used in association with presentations other than influenza/ILI and complications related to this. Ascertainment of clinical deterioration specifically related to the index influenza/ILI consultation would require analysis of free-text entries. However, this was not feasible with the resources available for this study.
It was not possible to address confounding due to severity of the index influenza/ILI episode, as data on clinical features relating to illness severity, including vital sign measurements, indicators of respiratory distress and duration of illness are not consistently coded in CPRD. We were also unable to adjust our analyses for additional social determinants such as access to health care and ethnicity due to lack of available data. Although we had intended to adjust our analyses relating to asthma according to the British Thoracic Society treatment step, we did not conduct this analysis because of difficulties with defining this variable reliably using the available data. Nevertheless, we were still able to conduct our pre-specified subgroup analysis examining asthma requiring treatment with inhaled corticosteroids or other preventer therapies as a risk factor.
Implications for clinical practice and further research
Primary care services should target children with asthma and neurological conditions when delivering interventions to prevent influenza and influenza-related clinical deterioration. Although asthma requiring regular preventer therapy is already highlighted as a risk factor [2], clinicians should also assess risk in other children with asthma, particularly high users of short-acting bronchodilators who may have poor disease control and hence also be at greater risk [Reference Maeda23]. Children with epilepsy should also be highlighted as a risk group. In our study, 85% of children with neurological conditions had epilepsy. However, epilepsy is not mentioned in current risk group definitions [2, 3], and is less commonly recognised by clinicians as a risk factor [Reference Smith24].
Nevertheless, most influenza-related complications still occur in children who do not have known risk factors [Reference Hardelid25]. Influenza vaccination is therefore still important in these children. Based on recommendations from the Joint Committee on Vaccination and Immunisation [7], the UK introduced a universal childhood seasonal influenza vaccination programme in 2013/14, starting with children aged 2 and 3 years, and extending up to children aged 8–9 years (i.e. year 4 of school) since winter 2017/18 [26]. However, effectiveness of the 2015/16 seasonal influenza vaccination was only around 58% in children aged 2–17 years in the UK [Reference Pebody27]. Furthermore, seasonal influenza vaccination rates in children with ‘at-risk’ conditions have not improved from around 40% since 2013/14 [Reference Rajaram28] and may be even lower during an influenza pandemic due to the time needed to develop and implement a suitable vaccine. Primary care clinicians may therefore need to consider more readily available treatments such as antibiotics and antiviral medications, especially given our observation that at least half of complications and hospitalisation occur within 1 day of initial presentation.
Strategies to inform efficient use of antibiotics and antivirals may include validated clinical decision rules involving use of point-of-care tests for inflammatory markers such as C-reactive protein [Reference Verbakel29] and potential respiratory pathogens including influenza [Reference Brendish30]. Further research is needed to inform efficient and cost-effective implementation of such strategies.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0950268818000353.
Acknowledgements
The authors would like to thank Lai Kin Cheah, Antonina Santalova and Bob Vause from the DEC Oxford Diagnostics PPI/E/P Group for their input with designing and reporting the findings of our study. The authors would also like to thank Dr Benjamin Feakins for providing us with Read codes for diabetes mellitus. This paper presents independent research funded by the National Institute for Health Research School for Primary Care Research (project no. 268). The views expressed are those of the authors and not necessarily those of the NIHR, the NHS or the Department of Health. The DEC Oxford Diagnostics PPI/E/P Group is supported through the NIHR Diagnostic Evidence Co-operative Oxford at Oxford Health Foundation Trust (award number IS_DEC_0812_100).
Declaration of interest
None.





