INTRODUCTION
Herpes zoster, caused by re-activation of the varicella zoster virus (VZV) commonly occurs in older adults and causes substantial morbidity. The incidence and severity of zoster is thought to increase with decreasing cell-mediated immunity and studies have shown consistently that the risk of zoster increases with age, particularly beyond 50 years [Reference Hope-Simpson1, Reference Thomas and Hall2] and also with specific immunosuppressive conditions [Reference Langan3, Reference Buchbinder4]. There is more limited evidence regarding the role of other factors. Some have suggested that zoster incidence may differ by sex [Reference Fleming5], ethnicity [Reference Thomas and Hall2, Reference Schmader6, Reference Zhang7], contact with children [Reference Thomas, Wheeler and Hall8], other illnesses [Reference Thomas and Hall2], and long-term stress or depression [Reference Schmader6, Reference Irwin9], but few studies have looked at a comprehensive range of characteristics in the same population with adjustment for multiple factors [Reference Weitzman10].
A live-attenuated VZV vaccine has been shown to reduce zoster risk in adults aged ⩾60 years by 51% and post-herpetic neuralgia by 66·5% [Reference Oxman11]; however, waning efficacy has been described with vaccine efficacy beyond 5 years uncertain [Reference Schmader12]. Some countries have recommended this vaccine be included in their adult immunization schedules although supply issues have delayed its introduction. In Australia, where this study was conducted, the vaccine was not readily available, even in the private sector, until 2013 although it was judged cost-effective for public funding in 2008. Given the challenges and costs associated with mass vaccination of adults, we conducted this study to estimate the incidence of herpes zoster, according to different characteristics, in a large unvaccinated general adult cohort. Our aim was to provide evidence to better understand the epidemiology of zoster, as well as to inform priorities for adult vaccination.
METHODS
The 45 and Up Study is a population-based cohort of over 267000 adults aged ⩾45 years in the Australian state of New South Wales (NSW). Detailed information on the cohort has been published elsewhere [Reference Banks and Redman13]. Briefly, adults resident in NSW were randomly selected from the Australian Medicare database, which has virtually complete coverage of the entire resident Australian population, and invited to take part in the study. Individuals completed a questionnaire which included items on socio-demographic characteristics, lifestyle and health (see: www.saxinstitute.org.au/our-work/45-up-study/questionnaires/) and provided consent for long-term follow-up, including linkage to administrative health records.
Participants had their survey data linked deterministically, using the Medicare Australia database, to their universal health insurance claims data [Pharmaceutical Benefits Scheme (PBS) and Medicare Benefits Schedule (MBS)]. They were also linked to state-wide hospitalization, cancer, infectious diseases and death registrations, using probabilistic matching of their name, date of birth and address. Probabilistic matching is highly accurate (false-positive and false-negative rates <0·4%) [14]. PBS data include a record of all medications prescribed and dispensed where the cost is above a threshold value. The date of prescription and supply, and exact formulation are recorded. Data were available from August 2004. The hospitalization data included a record of all hospitalizations in NSW including the admission date, the main diagnosis and up to 49 additional coded diagnoses related to the admission. Diagnoses were coded according to the International Classification of Diseases – version 10 (ICD-10) [15]. Records were available from July 2000. The cancer registrations include a record of all cancers diagnosed in NSW from 1994 coded according to ICD-10, and the date of diagnosis. The death records included a record of all deaths in NSW and the date of death. We had complete records for study participants from the PBS, hospitalization and death databases up to 31 December 2011.
We classified a participant as having a herpes zoster diagnosis if they linked to records of either a specific treatment for zoster in the PBS database, or a hospitalization with an ICD-10 code of ‘B02 – zoster’ in either the first (principal) or second diagnostic field. In Australia, antiviral medication (either acyclovir, valacyclovir or famciclovir) is recommended for treatment of zoster [Reference Dworkins16, 17]. A special ‘authority’ prescription needs to be obtained by the treating practitioner and the indication must be specifically for treatment of zoster (PBS codes: 1052J, 8002E, 8064 K, 8897G). These PBS records are therefore highly specific for zoster diagnoses and this method of ascertainment (prescription records) has been used in other epidemiological studies [Reference Langan3, Reference Zhang7, Reference Stein18, Reference MacIntyre, Chu and Burgess19]. Hospitalization data are less specific [Reference Yawn, Wollan and Sauver20]. Based on published data regarding the sensitivity and specificity of zoster codes in hospitalization data [Reference Jackson, Reynolds and Harpaz21] and our own inspection of the records, we classified a hospitalization if either the primary or secondary diagnosis fields contained an ICD-10 code for zoster. For the date of diagnosis we assigned the date of prescribing of the treatment, or the date of hospital admission, or whichever came first if there were links to both types of records.
To estimate the incidence and relative risks of zoster according to different characteristics hypothesized to be associated with zoster, we used data that were self-reported by participants at recruitment (e.g. socio-demographics) or data from one of the linked health records (e.g. immunosuppressive disorders or cancer diagnoses).
Ethical approval
All participants provided informed consent to be included in the study. The linkage study was approved by the NSW Population and Health Services Research Ethics Committee (HREC/10/CIPHS/97) and the 45 and Up Study was approved by the University of New South Wales Human Research Ethics Committee (no. 10186).
Analyses
We excluded study participants who were Department of Veterans Affairs card holders (n = 6323) as they can receive zoster treatment through an alternate scheme to the PBS, those with invalid dates of study entry or dates of birth (n = 26) and those with a zoster diagnosis prior to recruitment (n = 5854). Person-years of follow-up were calculated from study entry date to the first diagnosis of zoster, death, or 31 December 2011, whichever came first. Incidence rates were examined by sex and 5-year age groups according to attained age. Cox proportional hazards models with time updating of age were used to estimate relative risks of zoster according to different characteristics [Reference Thomas and Hall2, Reference Ultsch22]. Characteristics included sex, household income, married or living with a partner, having had children, education, area of residence [23], skin colour, country of birth (according to those with a known later onset of varicella infection), smoking, body mass index (BMI), alcohol consumption, frequency of vigorous physical activity, time spent outdoors, attending cancer screening programmes (mammography, prostate specific antigen testing or bowel cancer screening), use of supplements/vitamins, self-reported history of doctor-diagnosed asthma, cardiac disease, stroke, diabetes, depression/anxiety and hay fever. The level of physical limitation was assessed using the Medical Outcomes Study – Physical Functioning Score [Reference Hays24]. This is a 10-item standard questionnaire asking about whether an individual's health limits their ability to undertake physical activities including walking, climbing stairs, bending, lifting, dressing, etc.; participants are scored for each physical activity item based on whether they report being limited a lot, a little or not at all and the scores are combined to give an overall estimate of physical functioning with those not limited in any activities classified as ‘none’ and standard cut-points for each level of physical limitation, minor, moderate or severe.
Individuals were also classified according to whether they had a recent record of an immunosuppressive condition or cancer. An immunosuppressive condition was defined if individuals linked to a HIV registration, a PBS record of an immunosuppressive medication, or a hospitalization in the year prior to study entry for any one of a list of potentially immunosuppressive conditions including transplants or conditions that may result in administration of high-dose steroids (see Supplementary Appendix). Cancer was recorded if there was a record in the 5 years prior to study entry of a cancer registration or hospitalization with a primary diagnosis of cancer, except non-melanoma skin cancer (ICD-10 codes C00-C97 excluding C44).
All factors were initially examined in an age- and sex-adjusted model and then examined together in a multivariate model. Factors were included in the multivariate model if they were associated (P < 0·05) with zoster in the minimally adjusted model, or they were considered a priori as potential confounders. We also re-ran the multivariate analyses using only the outcome of hospitalization for zoster as a measure of severe disease. Subgroup analyses were conducted by age group (<60, 60–74, ⩾75 years), sex, area of residence (major city vs. regional/remote), and by measures of health service access (attending cancer screening programmes and taking supplements). Attained age was used as the underlying time variable in regression models. All analyses were conducted using SAS v. 9.2 (SAS Institute Inc., USA).
RESULTS
There were 255 024 participants included in analyses. At recruitment, 47·4% were aged 45–59 years, 37·1% were aged 60–74 years and 15·5% were aged ⩾75 years. Of participants, 1% (n = 2472) had a record of an immunosuppressive condition in the year prior to enrolment, 6% (n = 15 302) had a cancer diagnosis and 15% (n = 37 676) had severe physical limitations according to the standardized scale. There were 497 classified with both immunosuppression and cancer and 1143 classified with immunosuppression and severe physical limitation.
Over 940583 person-years of follow-up, 7771 participants had a zoster diagnosis of whom 253 (3·3%) were hospitalized. Of those hospitalized, 74% (n = 187) had zoster as the principal diagnosis. Overall incidence increased with age from 4·4/1000 person-years in those aged 45–49 to 12·1/1000 person-years in those aged >80 years. The increase was mostly observed between the ages of 45–75 years and across almost all age groups women had significantly higher rates than men (Fig. 1). For hospitalizations, the incidence was 0·01/1000 person-years in 45–49 year olds increasing sharply to 0·9 in those aged over >80 years (Fig. 2). Differences between men and women were less conspicuous in the hospitalization data.

Fig. 1. Incidence of zoster diagnoses per 1000 person-years by attained age and sex.

Fig. 2. Incidence of zoster hospitalization per 1000 person-years by attained age and sex.
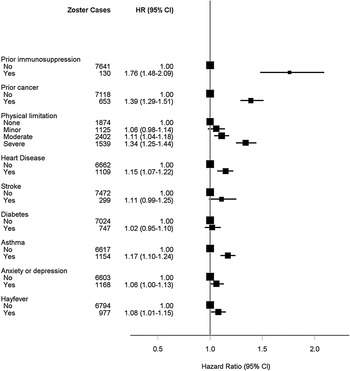
The age- and sex-adjusted analyses are shown in Figure 3(a–c). The greatest age- and sex-adjusted hazard ratios (HR) for zoster were found in those with a recent immunosuppressive condition (HR 1·76, 95% CI 1·48–2·09). In multivariate analyses, the relative risks for most of the characteristics identified in the age- and sex-adjusted analyses remained statistically significant (Fig. 4) with little attenuation. The risk of zoster remained greatest in those with a recent immunosuppressive condition [adjusted HR (aHR) 1·58, 95% CI 1·32–1·88, P < 0·0001). Other characteristics associated with higher risks included female sex (aHR 1·36, 95% CI 1·30–1·43, P < 0·0001), prior cancer diagnosis (aHR 1·35, 95% CI 1·24–1·46, P < 0·0001) and severe physical limitation compared to no limitation (aHR 1·33, 95% CI 1·23–1·43, P < 0·0001). When we restricted the outcome definition to hospitalizations for zoster only, i.e. focusing on risks for more serious disease, the only factors from the multivariate model that remained significant were immunosuppressive condition (aHR 3·78, 2·18–6·55, P < 0·0001), recent cancer diagnosis (aHR 1·78, 1·24–2·56, P = 0·002) and physical limitation (aHR 2·50, 1·56–4·01, P = 0·0001 and aHR 1·65, 1·05–2·59, P = 0·03 for, respectively, severe and moderate limitation compared to reporting no limitations).

Fig. 3a. Age- and sex-adjusted hazard ratios for zoster according to sociodemographic characteristics. * Includes Bangladesh, India, Malaysia, Pakistan, Singapore, Sri Lanka and Caribbean and Central American countries classified by the Standard Australian Classification of Countries.

Fig. 3b. Age- and sex-adjusted hazard ratios for zoster according to potentially modifiable characteristics. (* See Methods section.)

Fig. 3c. Age- and sex-adjusted hazard ratios for zoster according to illness and disability.

Fig. 4. Factors associated with an incident diagnosis of zoster, adjusted for all other factors in model and age.
Subgroup analyses (results not shown) did not demonstrate substantial differences compared to the main analyses. In particular the association of severe physical limitation with zoster risk remained significant in those more likely to be accessing health services, i.e. for those with severe physical limitation vs. none; those living in major cities (aHR 1·28, 95% CI 1·15–1·43); those attending cancer screening programmes (aHR 1·30, 95% CI 1·20–1·41); and those using supplements (aHR 1·27, 95% CI 1·15–1·41). We also found that the effect of having a severe physical limitation increased the risk of zoster when combined with other risk factors. For example, compared to those without severe physical limitations, cancer or immunosuppression, fully adjusted HR for zoster in those with severe physical limitations alone was 1·24 (95% CI 1·16–1·33), for those with immunosuppression or cancer 1·34 (95% CI 1·21–1·47) and for those with severe physical limitations and immunosuppression or cancer 1·85 (95% CI 1·61–2·12).
DISCUSSION
Our study identified a number of characteristics, other than age, that were associated with the incidence of zoster. Some factors, such as female sex [Reference Fleming5, Reference Opstelten25, Reference Tseng26], skin colour [Reference Schmader6, Reference Tseng26], smoking [Reference Schmader6], immunosuppressive conditions, in particular HIV [Reference Thomas and Hall2], recent cancer [Reference Weitzman10, Reference Habel27] and other chronic diseases [Reference Tseng26, Reference Forbes28] have been described by other authors. More novel findings included the 30% increase in risk of a diagnosis in individuals with severe physical limitations and the more than twofold risk of hospitalization, taking into account age and other comorbidities. These findings have important implications for who potentially could be prioritized to receive vaccination.
The strengths of our study include the large sample size, prospective design and ability to mutually adjust analyses for multiple potential confounding factors, including measures of health service use, income and education. Our ascertainment of a zoster diagnosis was based primarily on a prescription record of a highly specific treatment. Not all cases of zoster would be diagnosed and result in treatment so this method may have underestimated rates. It is also possible that treatment uptake may differ by age, leading to differential ascertainment. However, compared to published data from eight [Reference Weitzman10, Reference Oxman11, Reference Stein18, Reference Ultsch22, Reference Opstelten25, Reference Tseng26, Reference Chiappe29, Reference Insinga30] other large sources reporting zoster incidence in unvaccinated populations, which used methods ranging from clinician confirmation of self-report in a clinical trial setting [Reference Oxman11], physician-ascertained outpatient data [Reference Ultsch22] and general practice surveillance systems [Reference Chiappe29], we found our estimates of age-specific incidence to be consistent (see Fig. 5). Relying on zoster treatment to ascertain our outcome may be biased by access to health services. We found a higher incidence of zoster in those who attended cancer screening and those taking supplements and a lower incidence in those living in regional and remote areas, consistent with a health service access effect. However when we adjusted for these factors in the multivariate analyses there was limited attenuation of the hazard ratios obtained from the analysis with minimal adjustment (age and sex only; Fig. 3) suggesting that these factors do not substantially confound the associations described. It is also possible, given the multiple risk factors examined, that spurious associations may arise; however, our main results are consistent with other studies.

Fig. 5. Age-specific incidence of zoster diagnoses; comparison of 45 and Up Study diagnoses (black symbols) with other published research. (Diagnoses based on linkage to a zoster-specific treatment of ICD-10 coded hospitalization record.)
With the widespread vaccination of children through national varicella immunization programmes, there is substantial interest in whether environmental exposure may boost the immune response in previously infected adults to prevent future episodes of zoster. Exposure of adults to VZV through contact with infectious children has been proposed [Reference Thomas, Wheeler and Hall8] although a recent review suggested inconsistencies in the evidence [Reference Ogunjimi, Van Damme and Beutels31]. Data from our study did not appear to support this immune boosting theory. We found no difference in zoster risk in those who had children (although this was a crude measure of exposure) and zoster risks were consistently higher for women than men. In a cohort of this age, it is likely women would have greater exposure to young children and hence VZV than men.
The increased risks of zoster in those with immunosuppression or cancer are consistent with a hypothesis of decreasing cellular immune function [Reference Dworkins16]. It has been suggested that for asthma the increased risk may be due to use of inhaled corticosteroids [Reference Forbes28], although we did not have information to specifically examine corticosteroid use (oral or inhaled) in study participants. Our finding of a lower risk of zoster in current smokers (compared to non-smokers) has been reported in other observational studies and lower risk has also been described in racial groups with darker skin colour [Reference Schmader6]. There is, however, limited data to support a causal pathway and potential non-causal explanations for our findings include differences in health service access and ease of diagnosis (relative to skin colour). For example, compared to non-smokers, risks in current smokers were lower, and those in past smokers were increased. Current smokers are generally less likely to access healthcare while past smokers may be more health-seeking and have more comorbidities than current smokers and non-smokers, leading to the associations that we observed.
The increased risk of zoster and hospitalization for zoster in those with greater physical limitation is a novel finding. In this study, physical limitation was measured at the time of study recruitment using a standardized scale that combines a number of physical activities such as distance able to be walked, stair climbing and activities of daily living (e.g. bathing, dressing, bending, lifting) [Reference Hays24]. It is possible that the association with greater health-related physical limitations may be explained by an underlying decrease in immune function in individuals with chronic disease or disability [Reference Castle32]. It is also possible that we observed this association because those with greater physical limitations are more likely to access health services, resulting in a greater likelihood of a diagnosis of zoster. However, there was minimal attenuation of risk in the multivariate analysis, and the magnitude of risk remained similar when examined in subgroups restricted to those who were more likely to access services such as those attending cancer screening programmes, suggesting such a bias is unlikely.
The absolute risk estimates of herpes zoster from our study suggest that while incidence rises quite consistently with increasing age to about 75 years, the rise in zoster hospitalizations has a more exponential pattern with a rapid increase in those aged >70 years. Other than age, the principal risk factors identified included immunosuppressive condition, female sex, recent cancer, and severe physical limitation; and for zoster hospitalizations immunosuppression, cancer and severe physical limitation were important predictors. The live-attenuated zoster vaccine is contraindicated in those with specific immunosuppressive disorders and in pregnancy [33]. While there are limited clinical trial data demonstrating vaccine efficacy in other vulnerable subgroups identified in our study, for example those with cancer or physical limitations, some recent large observational population-based studies suggest that the vaccine is safe and effective in preventing zoster in those with chronic diseases and in the very elderly [Reference Langan3, Reference Zhang7, Reference Morrison34]. Given the high risk of hospitalization for zoster in the very elderly and also those with predisposing conditions such as prior cancer or severe physical limitations, further evaluation of the effectiveness and cost-effectiveness of zoster vaccine in the risk groups identified here should be a priority.
SUPPLEMENTARY MATERIAL
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/S0950268814003653.
ACKNOWLEDGEMENTS
This research was completed using data collected through the 45 and Up Study (www.saxinstitute.org.au). The 45 and Up Study is managed by the Sax Institute in collaboration with major partner Cancer Council NSW; and partners: the National Heart Foundation of Australia (NSW Division); NSW Ministry of Health; beyondblue; Ageing, Disability and Home Care, Department of Family and Community Services; the Australian Red Cross Blood Service; and Uniting Care Ageing. We thank the many thousands of people participating in the 45 and Up Study. The NSW Centre for Health Record Linkage conducted the linkage of the datasets. The NSW Ministry of Health provided de-identified data to the researchers and the Pharmaceutical Benefits data was made available by the Department of Human Services.
This work was supported by the Australian National Health and Medical Research Council (NHMRC grant no. 1048180) and the Clive and Vera Ramaciotti Foundation. The funders had no input into the study design, analysis, interpretation, writing nor decision to submit for publication. A.T.N., B.L., E.B., J.M.K. are recipients of NHMRC fellowships.
DECLARATION OF INTEREST
B.L. has shares in bioCSL the distributor of zoster vaccine in Australia. C.R.M. has done investigator-driven research on zoster funded by Merck (USA), a Merck randomized controlled trial of zoster vaccination and sits on the WHO SAGE varicella zoster virus working group. A.E.H. has received grant funds for investigator-driven research from GSK and Sanofi Pasteur. A.T.N. has in the past received research funding paid to his institution from a vaccine manufacturer for other previous projects. All other authors have no competing interests to declare.