INTRODUCTION
Variations in the numbers of cattle shedding E. coli O157 and the levels at which they shed the bacteria in their faeces have been reported from prevalence surveys and longitudinal studies [Reference Karmali, Gannon and Sargeant1, Reference Meyer-Broseta2]. In particular, individuals shedding at very high levels, >10000 c.f.u./g, have been identified and are classified as super-shedders [Reference Chase-Topping3]. The causes of increased prevalence and super-shedding have been investigated previously but are likely to be multi-factorial, and an increased probability of shedding has been demonstrated in animals housed with super-shedders [Reference Cernicchiaro4, Reference Cobbold5]. Risk factors for increased shedding are likely to cover a wide range of animal, environmental and pathogen factors and interactions between them are likely to be complex.
Management strategies, such as nutrition [Reference Callaway6–Reference Russell, Diez-Gonzalez and Jarvis8], have been the focus of some previous research with the goal of identifying intervention strategies for pre-slaughter controls. Few studies have noted that animal factors may influence shedding [Reference Khaitsa9, Reference Chase-Topping10] but these factors have not been thoroughly tested. Variations in shedding by different breeds have been investigated; however, results are often unsubstantiated statistically due to small sample sizes and confounding factors [Reference Irshad11]. Body weight has been noted to have an effect on the probability of shedding but results are contradictory [Reference Khaitsa9, Reference Smith12, Reference Dargatz13].
Seasonal changes in E. coli O157 shedding are well documented with higher occurrence during summer months [Reference Ando14–Reference Barkocy-Gallagher16], widely believed to be associated with temperature. The assumption that increased ambient temperature is a contributing factor to increased shedding has not been well substantiated. Increased mean ambient temperatures have been linked to increased E. coli O157 shedding [Reference Kondo15] and modelling has been undertaken to understand the effect of temperature on environmental contamination [Reference Gautam17]. Edrington et al. [Reference Edrington18] provided evidence for an effect of day length on shedding based on experimental work. Kondo et al. [Reference Kondo15] observed an effect of rainfall but this was not significant compared to other factors. Smith et al. [Reference Smith12] noted an increase in shedding associated with muddy conditions.
Many of these previous investigations have considered only a few risk factors and therefore may not provide an accurate assessment of all interactions. Inclusion of a more comprehensive range of potential risk factors is necessary due to the likely complex interactions which influence shedding.
A study by Chase-Topping et al. [Reference Chase-Topping10] investigated risk factors for super-shedding and concluded that the phage type of the bacteria was significantly associated super-shedding. The type of animal, particularly breeding cattle, and stress associated with transport and weaning were also identified as risk factors but no environmental factors were noted as risk factors. Few other studies have considered risk factors for super-shedding.
The aims of this longitudinal study were to identify factors associated with increased shedding and super-shedding in a more comprehensive manner than has been undertaken previously, and to identify if different factors influence the level of bacterial shedding by individuals. The mechanisms driving increased prevalence and super-shedding are poorly described and a greater understanding of these may provide intervention strategies to reduce E. coli O157 shedding.
MATERIALS AND METHODS
Animals and sampling
A cohort of 52 home-bred replacement dairy heifers was sampled weekly between September and December 2012 and between January and February 2013. Animals were maintained on ~15 acres pasture in accordance with standard University of Sydney farm practices and received supplementary feed of hay and high protein pellets ad libitum. Dam access was available for water. Recto-anal mucosal swabs (RAMS) and faecal samples were obtained as described by Khaitsa et al. [Reference Khaitsa9] with slight modifications. Briefly, RAMS were obtained prior to faecal sampling and placed into 10 ml buffered peptone water (BPW). About 10 g faeces was taken using digital rectal palpation and sealed into a zip-lock bag. All samples were placed on ice and transported to the laboratory for processing within 4 h of collection. The RAMS sample and faecal sample taken from each heifer on each occasion represented one animal sampling point (ASP).
Ethical standards
The use of animals in this study was approved and monitored under University of Sydney Animals Ethics Committee Protocol number N00/4-2011/3/5487.
Animal data
For each ASP taken the animal number was recorded, along with the body condition score of the heifer, faecal consistency and hide cleanliness. Body condition was scored from 1 (emaciated) to 5 (obese) using standard methods described by DAFF [19]. Faecal consistency was scored as described by Alberta Dairy Management [20] from 1, representing a liquid consistency, to 4, representing a dry sample. Hide cleanliness was scored following the guidelines of the Food Standards Agency [21], where 1 = clean and dry, and 5 = filthy and wet. Hide contamination was assessed over the perineal region and back legs rather than the entire animal.
From herd records, date of birth and breeding information were obtained.
Detection of E. coli O157
Samples were tested for the presence of E. coli O157 by direct faecal culture (DFC) and immunomagnetic separation (IMS) as described previously [Reference Williams22]. Briefly, 10 g of faeces in 90 ml BPW was mixed thoroughly and 100 μl was directly cultured onto sorbitol MacConkey agar supplemented with cefixime and tellurite (CT-SMAC). The faecal dilutions and RAMS in 10 ml BPW were then enriched at 37°C for 6 h. Enriched cultures were stored overnight at 4°C and ASPs which did not yield E. coli O157 by DFC were tested by IMS on faeces and swabs following the manufacturer's instructions. Briefly, a 1 ml aliquot of the enrichment culture and 20 μl Dynabeads® (Invitrogen Dynal, Norway) were mixed for 15 min and washed three times with IMS wash buffer (PBS/Tween). The washed beads were re-suspended in 100 μl IMS buffer and 50 μl of this suspension was plated onto CT-SMAC and incubated overnight at 37°C. Aliquots of direct faecal broths and enriched faecal and RAMS broths were stored at −80°C with 20% glycerol.
Confirmation and recovery of E. coli O157 isolates
CT-SMAC plates from DFC and IMS were incubated overnight at 37°C and suspect (straw-coloured) colonies were isolated by streaking onto non-selective agar for confirmation using an O157 latex agglutination test (Oxoid, UK). Isolates were also tested by PCR for further confirmation of serotype by rfbEO157 [Reference Bertrand and Roig23] and 16 s rRNA [Reference Wang, Clark and Rodgers24], the toxin genes stx1 [Reference Wang, Clark and Rodgers24] and stx2 [Reference Bai, Shi and Nagaraja25], and additional virulence factors eae and hlyA [Reference Bai, Shi and Nagaraja25]. One isolate from each ASP was tested.
Environmental and climate data
Day-length data were obtained from Geoscience Australia (GA) records [26]. Pasture growth was estimated using remote sensing by ‘pastures from space’ (CSIRO) [27], and was recorded as average growth for Camden shire per week. Maximum temperature, total rainfall, total solar exposure (SE) and relative humidity (RH) at 09:00 and 15:00 hours were obtained for each day throughout the trial from the Bureau of Meteorology (BOM) [28]. The BOM weather station from which recorded data was obtained was located <3 km from the herd location.
Statistical analysis
Risk factors were tested at two outcome levels, E. coli O157 positive by any test (⩾1 c.f.u./g) or super-shedding as determined by DFC (⩾10000 c.f.u./g), to assess factors for shedding and super-shedding independently. Risk factors were grouped as animal factors (body score, hide cleanliness, faecal consistency), environmental factors (day length, pasture growth) and climate factors (temperature, rainfall, SE, RH).
For climate data, ‘risk factor’ levels were arbitrarily assigned for each factor and a count made of the number of times these levels were reached in the 3, 5, 7 and 14 days preceding each sampling date.
Temperature risk factors were arbitrarily assigned as days ⩾30°C, ⩾37°C or ⩾40°C. Rainfall risk factors considered were number of days of any recorded rain and number of days of rain ⩾10 mm. High RH data from 09:00 and 15:00 hours was combined to provide the total number of days when RH was ⩾80%, ⩾90% or ⩾95%. Arbitrary risk levels of ⩾20 mJ/m2, ⩾25 mJ/m2, ⩾30 mJ/m2 were assigned to the SE data, and the number of days in which these levels were exceeded prior to sampling counted.
Each potential risk factor was initially assessed for association with E. coli O157 shedding or super-shedding individually. Summary statistics and correlation coefficients were calculated in Microsoft Excel (Microsoft Corp., USA).
Logistic regression (χ 2) was undertaken in GenStat 14th edition (VSNI, UK) and factors significant at 5% were investigated further. Odds ratios (ORs) were used to assess the size of the climate effects on shedding and super-shedding. For each of the four climate factors the classification method indicating the largest and most consistent effects was selected for further analysis.
A general linear mixed model (GLMM) was used to test the significance of factors in each group including animal ID as a random effect. Factors not significant at 5% by the Wald test were removed from further analysis.
The effects significant in these three group models were then combined to produce final GLMMs of the significant factors influencing shedding and super-shedding. Where a large number of fixed effects were tested in a model or where there was missing data which prohibited fitting the model, data categories were collapsed. All GLMMs were run in GenStat.
RESULTS
Recovery of E. coli O157
A total of 933 ASPs were collected during the trial, including three duplicate ASPs when a heifer was sampled twice on the same date. The three duplicate samples were included in analysis and final models included heifer number. Faeces were not obtained for 37 ASPs.
A total of 149 (16·6%) faecal samples were identified as positive by direct culture of 896 ASPs tested. Of these samples, 32 (3·6%) were enumerated as shedding ⩾10000 c.f.u./g. The 32 incidences of super-shedding detected were from 24 heifers, of which 19 were detected as super-shedding once only. A further 267 (28·6%) ASPs were detected as positive by IMS of RAMS or faeces to give a total of 416/933 (44·6%) positive ASPs (Table 1).
Table 1. Results of E. coli O157 shedding and super-shedding within the cohort over the duration of the sampling period (2012–2013)

Of the recovered isolates, 366 were tested by PCR. Fifty isolates could not be recovered at the time of testing to confirm virulence profile. All except two were positive for rfbEO157 and 16S rRNA. The 364 isolates confirmed as E. coli O157 by PCR were positive for stx2 and 360 were positive for stx 1. One isolate positive for rfbEO157 and both stx genes was negative for eae and hlyA, to give 259/366 (98·1%) positive isolates for all virulence genes tested.
Date of sampling
The date on which a sample was obtained had a significant association with the probability of E. coli O157 shedding (P < 0·001) but not on the occurrence of super-shedding (P = 0·853). This association can be clearly seen from the changing prevalence levels throughout the study. The mean prevalence was 44·6% (standard error of the mean = 5·4, 95% confidence interval = 33·1-56·1), ranging from 9·4% to 94·3% at individual sampling points. Sampling date was not included as a random factor in further analyses because the time-dependent effects of host, climate and environmental factor were the primary focus of this study.
Animal risk factors
A very small number of heifers were classified with hide cleanliness scores of 5 (filthy and wet, n = 4), or 4 (very dirty, n = 14) which represented ⩽5% of the total results. These results were combined with scores of 3 to create a category (scores ⩾3) of suitable size for analysis (n = 92). Hide cleanliness was not significantly associated with shedding (P = 0·892).
Body condition score (P < 0·001) and faecal consistency (P = 0·01) were demonstrated to be significant by logistic regression (χ 2), the effects are shown in Table 2. Inclusion of both fixed effects in a GLMM indicated that both body score (F statistic = 11·43, P < 0·001) and faecal consistency (F statistic = 4·39, P = 0·004) remained significant in this model. The interaction of body score and faecal consistency was not significant (F statistic = 0·31, P = 0·931).
Table 2. Effects of animal factors on the shedding of E. coli O157 in a cohort of 52 dairy heifers, identified by logistic regression.
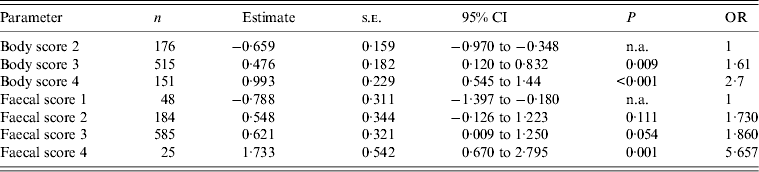
s.e. Standard error; CI, confidence interval; OR, odds ratio; n.a., not applicable as the reference level.
Logistic regression demonstrated an effect on super-shedding for hide cleanliness (P = 0·031) and faecal consistency (P = 0·044), as shown in Table 3, but not body score (P = 0·876). Inclusion of significant factors in a GLMM indicated both hide cleanliness (F statistic = 7·02, P < 0·001) and faecal consistency (F statistic = 826·4, P = 0·015) remained significant; however, the GLMM could not be resolved to test for interactions between these factors.
Table 3. Animal factors demonstrating a significant effect on super-shedding of E. coli O157 in a cohort of 52 dairy heifers, identified by logistic regression

s.e. Standard error; CI, confidence interval; OR, odds ratio; n.a., not applicable as the reference level.
Environmental risk factors
Day length varied between 684–866 min during this period of the trial. Pasture data was presented as the average per week and ranged from 0 to 67 kg/ha per day.
The large range of data values for these environmental variables and the nature of the variables (continuous) indicated the need to categorize the data. Pasture growth was therefore categorized as no growth, low growth (1–29 kg/ha per day) or high growth (⩾30 kg/ha per day). Logistic regression (χ 2) indicated significance of pasture growth on E. coli O157 shedding both as continuous (P > 0·001) and categorical (P < 0·001) data (Table 4). Pasture growth was not significant on super-shedding either as a continuous (P = 0·071) or a categorical (P = 0·186) variable.
Table 4. Effect of pasture growth and day length on E. coli O157 shedding in a cohort of 52 dairy heifers; both effects were tested as continuous variables and also were collapsed into three categories for testing

s.e. Standard error; CI, confidence interval; OR, odds ratio; n.a., not applicable as the reference level.
The consistent pattern of changing daylight hours over the duration of the trial did not match the erratic results of shedding. Analysis did indicate a significant association between E. coli O157 shedding and daylight hours. This effect was demonstrated both by analysis as a continuous variable (P = 0·01) and when collapsed into categories (P < 0·001) of low (734–776 min), medium (777–817 min) and high (818–862 min) although the results were not consistent (Table 4). No effect of day length on super-shedding was demonstrated either as a categorical variable (P = 0·693) or as a continuous variable (P = 0·375).
A GLMM including pasture growth and day length indicated both remained significant (P < 0·001) when tested as continuous and categorical variables.
Climate risk factors
Data for maximum daily temperature, amount of rainfall, total SE and RH at 09:00 and 15:00 hours was obtained for each day during the sampling period to produce an extensive dataset. The maximum temperature ranged from 17·0°C to 46·4°C, rainfall ranged from 0 to 125·2 mm/day, and SE ranged from 3·1 to 34 mJ/m2. RH ranged from 10% to 99%. High RH (⩾80%) was more common at 09:00 hours (n = 26) than at 15:00 hours (n = 6) over the duration of the trial. These data were combined to give the total number of days when RH ⩾80% (n = 28). No strong interactions were detected between the climate factors (data not shown), therefore each factor was analysed individually.
Extensive investigations of the climate data were undertaken to identify optimal analysis methods. This included assessing the means prior to sampling, categorizing data, the maximum readings and readings at a given point prior to sampling. The identification of specific risk factors and how often they occurred allowed for size and duration of climate effects to be included; but even with this simplified data extensive investigations were required to refine categorization. Correlations between each of these assigned risk factors and E. coli O157 detection or super-shedding indicated some associations between climate and shedding (Table 5). Logistic regression supported many of these associations and indicated non-significant factors which were dropped from further analysis. Generally, the strongest and most consistent associations were noted between rainfall and both shedding and super-shedding, and between RH and super-shedding.
Table 5. Correlations of assigned climate risk factors to E. coli O157 shedding and super-shedding in a cohort of 52 dairy heifers. Associations >0·5 (moderate) are shown in bold. Time-frame represents the number of days prior to sampling over which climate factors were assessed

RH, Relative humidity; SE, solar exposure.
* Associations not significant by logistic regression
The size of effects of each climate risk factor from those significant by logistic regression indicated the strongest and most consistent effects for classification for each factor. From this, the classification methods for further analysis were selected, along with consideration of the raw data. For example, a large effect of temperature exceeding 40°C in the 5 days preceding sampling was demonstrated; however, as this occurred on only one sample point the result should be treated with caution (data not shown). The climate classifications selected and the effects on E. coli O157 shedding and super-shedding are shown in Tables 6 and 7, respectively.
Table 6. Climatic associations with E. coli O157 shedding in a cohort of 52 dairy heifers, estimated by bivariate logistic regression. Levels are the number of days the arbitrary threshold was reached in the 7 days prior to sampling with the exception of temperature (T), which was counted over 14 days prior to sampling

s.e. Standard error; CI, confidence interval; OR, odds ratio; n.a., not applicable as the reference level; RH, relative humidity; SE, solar exposure.
Table 7. Climatic associations with E. coli O157 super-shedding in a cohort of 52 dairy heifers, estimated by logistic regression. Levels for each factor represent the number of days the threshold value for was reached in the 7 days preceding sampling. The exception was temperature (T), which was counted in the 3 days preceding sampling

s.e. Standard error; CI, confidence interval; OR, odds ratio; n.a., not applicable as the reference level; RH, relative humidity; SE, solar exposure.
Inclusion of all climate fixed effects in the GLMM for shedding could not be resolved for SE. Collapsing SE from categories used for logistic regression (Table 6) to categories of low (1 day ⩾20 mJ/m2), medium (3, 4, or 5 days ⩾20 mJ/m2) or high (6 or 7 days ⩾20 mJ/m2) provided a dataset suitable for analysis. The model of combined climate fixed effects indicated all associations remained significant for E. coli O157 shedding.
Dropping non-significant terms from the climate GLMM for super-shedding including all fixed effects indicated that only rainfall was significant with respect to super-shedding (P < 0·001).
Final model for E. coli O157 shedding detection
All animal factors, environmental factors and climate factors shown to be significant were made available for inclusion in the final model. Pasture growth and day length were included as categorical factors with three levels for each. Climate factors were included at the levels successfully tested in the climate model but the model could not be fitted for all effects. Rainfall and temperature were therefore collapsed from six to three levels each. Rainfall over the previous 7 days was classed as none, low (1–3 days rain) or high (4 or 5 days rain). Temperature over the previous 14 days was classed as low (<37°C), medium (1–2 days ⩾37°C) or high (3–5 days ⩾37°C). Sequentially dropping non-significant terms from this GLMM resulted in a model that included all climate factors together with pasture growth and body score (Table 8). All factors in this model were categorized at three levels.
Table 8. Results of the final multivariate model for E. coli O157 shedding in a cohort of 52 dairy heifers. This GLMM included all significant fixed effects with animal as a random effect. Climate factors were counted as the number of days a set value was exceeded in the previous 7 days, with the exception of temperature which was counted over 14 days. Results were categorized to three levels, with the exception of relative humidity which had three data levels only

Testing the effects of SE in an individual GLMM indicated that the probability of E. coli O157 recovery was 54% with low SE, 52% with medium SE and 33% with high SE.
Final super-shedding model
A GLMM of all individually significant factors could not be fit to the data due to the relatively small number of super-shedding events detected; several combinations of factors were not represented in the data. Data was therefore collapsed to provide each factor with three categorical outcomes. Dropping non-significant terms from the complete model identified hide contamination (P = 0·002), faecal consistency (P = 0·023) and rainfall (P < 0·001) to be significantly associated with super-shedding (Table 9).
Table 9. Results of the final multivariate model for super-shedding in a cohort of 52 dairy heifers. This GLMM included all significant fixed effects with animal as a random effect. Rainfall was measured as the number of days in the preceding 7 days in which rainfall ⩾10 mm was recorded

DISCUSSION
These study results indicated an increased probability of shedding with increasing body score (P = 0·029). Weight, body score and age are all likely to be related, and age has been reported to be associated with E. coli O157 shedding [Reference Ferens and Hovde29]. Specifically, shedding is most frequent in post-weaning calves and heifers [Reference Zhao30, Reference Hancock31], i.e. the age range of the cohort tested in this trial. In growing animals, age and weight may be more important than body score. However, in mature animals, weight fluctuations are likely to be less marked and body score may be a better indicator. Body score increased over the duration of the current study, as did age and weight. Van Donkersgoed et al. [Reference Van Donkersgoed, Graham and Gannon32] demonstrated no effect of body score on E. coli O157 shedding but the results of this analysis may be affected by the low number of positives detected (7·5% of 1247 samples). An effect of body weight has previously been demonstrated for E. coli O157 prevalence but the evidence is inconclusive. Dargatz et al. [Reference Dargatz13] noted reduced shedding in animals >317·5 kg entering a feedlot whereas Khaitsa et al. [Reference Khaitsa9] noted reduced shedding in feedlot animals weighing <408 kg. Further investigations into the interactions between body score, weight and age are therefore necessary to understand how these factors influence E. coli O157 shedding.
Faecal consistency was demonstrated to be associated with super-shedding (P = 0·023) and an effect of faecal consistency on detection was also noted although it was not significant in the final shedding model. Faeces type has previously been shown to affect E. coli O157 survival with higher survival in faeces from grain-fed as opposed to hay-fed cattle [Reference Lowe33]. All heifers in this study received the same diet; however, faecal consistency may be a marker for other faecal factors. No insight as to the direction of any association can be drawn from this result. Both the potential that E. coli O157 within the gastrointestinal tract of heifers affects the system causing changes to faecal consistency, and the alternative that changes in faecal consistency from any cause may affect the shedding of E. coli O157, are reasonable arguments. An alternative explanation for this association is that the relationship observed is based on increased detection from firmer faeces rather than actual increases in shedding. Firmer faeces with a higher dry-matter content may simply provide a larger effective sample size; as sample size has been previously shown to affect detection [Reference Omisakin34]. Strategies to reduce the moisture content of very liquid faeces may provide an option to increase the sensitivity of the tests and warrants further investigation.
An association was demonstrated between decreased hide cleanliness and increased super-shedding (P = 0·002). A previous study noted a positive correlation between E. coli O157 contamination of hides and faecal shedding, but did not assess hide cleanliness [Reference Elder35]. Heifers with dirty hides, including faecal matter and mud, which lick themselves could represent a simple mechanism of continuous infection which may include the ingestion of large numbers of bacteria consequently excreted in faeces. However, the association observed in this analysis of hide cleanliness should be viewed with some caution as very few animals exhibited very dirty hide scores over the duration of the trial.
Pasture growth was demonstrated to be associated with shedding and remained significant in the final model (P = 0·013). Diet and variations in nutrition have been associated with changes in E. coli O157 shedding [Reference Callaway6, Reference Jacob, Callaway and Nagaraja7] and changes in pasture growth would represent changes in available nutrition. Alternatively, the optimal conditions for pasture growth, such as ambient temperature and moisture availability, are also optimal for E. coli O157 growth which may provide ideal conditions for proliferation in the environment. A direct effect of pasture cannot be ruled out and the observed effect may be multi-factorial such that optimal growth conditions allow for an increase in environmental E. coli O157 and changes in pasture affects the passage of E. coli O157 through the heifers.
Results indicated an association between daylight hours and shedding within the cohort that was small and inconsistent (P = 0·01) and that did not remain significant in the final model. No association to super-shedding was observed. Increasing day length has previously been noted to increase E. coli O157 shedding [Reference Edrington18]. However, the trends observed in our results indicated that while day length may be associated with the ‘baseline’ level of shedding, the large variations in shedding during the trial period could not be explained by the gradual changes in daylight hours. This suggests that whilst daylight may have an effect on E. coli O157 shedding, other factors exert greater pressure on prevalence.
All climate factors tested were demonstrated to be associated with E. coli O157 shedding but only rainfall was demonstrated to be significant for super-shedding in the final model. Increased temperature preceding sampling was generally associated with increased prevalence and decreased super-shedding (Table 5) and a strong effect of temperature was noted in the final shedding model. Seasonality of E. coli O157 prevalence has been well documented previously with higher prevalence noted in warmer months [Reference Meyer-Broseta2, Reference Barkocy-Gallagher16, Reference Van Donkersgoed, Graham and Gannon32]. The assumption that seasonal changes are associated with changes in temperature is generally accepted but limited studies have supported this. One study has demonstrated the effect of mean temperatures 30 days prior to sampling [Reference Kondo15] and the effect has been demonstrated with modelling [Reference Gautam17]. Experimental storage of inoculated faeces has also shown increased survival of E. coli O157 at higher temperatures [Reference Lowe33]. Results of the current study indicate that higher ambient temperatures do have a positive effect on E. coli O157 shedding. The contradictory reduction in super-shedding observed with increased temperature may be indicative of small effects, the low numbers detected as super-shedding, or may be associated with the increased SE expected when temperature is very high.
Correlations indicated negative effects of SE. A reduction of environmental E. coli O157 associated with the UV effects of SE would provide a mechanism reducing exposure of cattle to E. coli O157. However, anomalous large, positive results associated with some levels of SE were observed by regression analysis and in the final model, SE was also demonstrated to have a positive effect on shedding. This changing of the direction of the association, known as Simpson's paradox, is likely due to the distribution of the data with respect to other climate factors.
A positive association between increasing rainfall and increased shedding and super-shedding was observed (Tables 5, 8, 9). Kondo et al. [Reference Kondo15] previously identified an association between rainfall and E. coli O157 prevalence and moisture is necessary for bacterial growth and replication. Effects of high rainfall were noted over a long time-frame, likely to be the result of persistence of moisture in the environment. This could therefore provide optimal conditions for E. coli O157 for a longer duration. Increased rainfall would also contribute to muddy conditions noted by Smith et al. [Reference Smith12] to increase prevalence, which may also be linked to decreased hide cleanliness noted to be associated with increased super-shedding.
A trend of increased shedding and super-shedding was noted with high RH, although not significant in the final super-shedding model. RH has not been previously investigated as a risk factor for E. coli O157 shedding, although has been considered with respect to E. coli O157 contamination of leafy vegetables [Reference Ding36–Reference Wang and Oh38]. High RH associated with higher moisture and temperatures could be expected to provide optimal conditions for E. coli O157 survival and replication in the environment. The mechanism of climate effects on environmental E. coli O157 is speculative and extreme climate effects may also effect a physiological response from animals which in turn affects shedding.
Between-animal variation has been well documented previously, often in parallel with descriptions of super-shedding [Reference Chase-Topping3] and has been supported by ex vivo experiments demonstrating differential adherence of E. coli O157 to intestinal epithelium from different animals [Reference Baines, Lee and McAllister39]. Little further investigation into the animal traits which may influence E. coli O157 carriage and shedding by cattle has been undertaken. The lack of research into the potential genetic traits of E. coli O157 ‘susceptibility’ will continue to be influenced by the difficulties encountered in this study, primarily sample size combined with a need for repeated measurements.
Results indicate that the effects of climate, pasture growth, and body score are significantly associated with E. coli O157 shedding. Previous studies have considered some of these factors on shedding, but the majority of studies have considered only one or two factors in isolation. The inclusion of multiple associations within the model will have reduced the statistical power of the analyses but the changes in results observed with progressive analysis of this data demonstrate the need for comprehensive analysis. The effects of other potential factors not considered may also have affected the results observed in this study. Effects on shedding are likely to be multi-factorial and several of these factors are likely to interact; therefore the final model presented should be taken as indicative of trends rather than a definitive model. Maintaining the cohort with consistent management conditions did not allow for analysis of management factors in the present study but reduced the number of confounding factors in the data which was analysed.
The reduced number of factors demonstrated to be associated with super-shedding may indicate that some factors have a larger effect on super-shedding but may also be attributable to lower significance due to the lower numbers of super-shedding detected. The identification of factors which may be associated with super-shedding may provide options for intervention strategies and therefore warrants further investigation.
CONCLUSIONS
Several risk factors were identified which were associated with E. coli O157 shedding and super-shedding. Of the environmental factors tested, rainfall had a positive effect on shedding and super-shedding. Temperature and relative humidity also had positive effects on shedding and these three climate factors are likely to be related. Differences between the final models for prevalence compared to super-shedding may indicate different mechanisms behind the outcomes. It should not be ruled out that the differences in these models indicate flaws in the results based on coincidence and the small number of super-shedding events detected.
Many of the interactions are difficult to untangle; however, some key factors which increase the risks of high prevalence and super-shedding have been identified. The trends observed indicate environmental conditions associated with warm, damp and likely muddy conditions in which E. coli O157 may thrive and increased spread of dirt and faecal matter may provide a mechanism for distribution within the herd. This mechanism may also be associated with animal factors of faecal consistency and hide contamination noted to be significant for super-shedding. Conversely, hot and dry conditions provide an environment in which E. coli. O157 struggles to survive. This conclusion indicates that E. coli O157 survival and replication within the environment is necessary to maintain a population within a bovine cohort, pointing to a mechanism of continuous infection compared to colonisation within cattle.
ACKNOWLEDGEMENTS
The authors acknowledge the technical assistance of Ms. Lechelle Van Breda throughout the study, and the assistance of Ms. Jessica Ann Sanchez with the PCRs. This study was funded by Meat & Livestock Australia Ltd, project number A.MFS.0257.
DECLARATION OF INTEREST
None.












