INTRODUCTION
Anthrax, caused by Bacillus anthracis, has a wide host range, but is primarily a disease of herbivores. Many farm and wild animals are infected, with high mortality [Reference Hanna and Ireland1–Reference Aikembayev5]. Wild carnivores can also become infected following consumption of animals that died of anthrax [Reference Lindeque and Turnbull6]. Birds are naturally resistant to anthrax [3], but humans can be infected by coming in contact with infected animals or with by-products of animal origin harbouring the spores of the organism [4]. Three forms of anthrax, i.e. cutaneous, pulmonary and intestinal, are seen in humans, the first form is predominant (>95%) [4, Reference Hugh-Jones7, Reference Macher8]. According to official data, the worldwide annual number of human anthrax cases ranges from 2000 to 20 000 [Reference Hugh-Jones7]. The most important procedure in order to protect humans from anthrax is controlling anthrax in animals.
The dormant spores of B. anthracis are extremely tolerant to harsh environmental conditions, enabling them to survive several decades [4]. Enhanced disease management in animals in developed countries has removed this threat. Nevertheless, anthrax outbreaks have been reported in the USA [Reference Mongoh9], Australia [Reference Durrheim10], Italy [Reference Fasanella11], Sweden [Reference Lewerin12] and many other European countries within the last 10 years at various frequencies [13]. Anthrax is sporadic or endemic in South Asian countries [Reference Vijaikumar, Thappa and Karthikeyan14–17] including Bangladesh. There was an increased number of anthrax outbreaks in domestic animals in the two most densely cattle-populated districts in the country, Pabna and Sirajganj, in the North-west, and 55 human cases were registered there in August–October 2009 [17]. At the same time of year the disease re-emerged in the same two districts in 2010, resulting in 288 human cases out of 607 recorded countrywide by 28 October 2010 [18, 19]. All the human cases reported in 2010 were of the cutaneous form. It is therefore vital to discover the risk factors in order to prevent a re-occurrence in the future. The mechanisms by which animals infect humans in Bangladesh are not well documented. The cattle in the two districts are kept predominantly in smallholdings, which is common in South Asia, but information on risk factors for anthrax in cattle in smallholdings in any part of the world is lacking. Here, we elucidate the risk factors associated with anthrax outbreaks in cattle.
MATERIALS AND METHODS
Geography of the study area
Bangladesh is divided into seven divisions, 64 districts and 481 sub-districts (upazilas) (an upazila is a lower administrative unit in Bangladesh). The study was conducted in the districts of Pabna and Sirajganj, in North-western Bangladesh. In 2009 all the human anthrax cases in Bangladesh were reported from these two districts, and all occurred during August–October [17]. Because of this, and because the first case of anthrax in 2010 was also reported from the same area these two districts were selected for study. These districts each comprise nine upazilas. The cattle population of Bangladesh is about 24·5 million [Reference Kamal20], 90% of which are nondescript indigenous and 10% cross-bred [Reference Kamal20]. According to the Bangladesh Department of Livestock Services (DLS), the cattle populations in these two districts are the densest in the country and the number of cross-bred animals much higher than any other part of the country. Green fodder is fed to cattle from November to April [Reference Kamal20]. The livelihoods of many people in these two districts depend on small-scale dairy farming, and the area is the major source of milk in Bangladesh.
The two districts have an indigenous type of cattle named Pabna, after the district [Reference Kamal20]. Along with crossbreds these cattle constitute most smallholders' herds. Being at the junction of two great rivers: Ganga (in India)/Padma (in Bangladesh) and Brahmaputra (in India)/Jamuna (in Bangladesh), before flowing as a single river (Padma) downstream (Fig. 1), the grazing fields are flooded each year. After October, the land drains and the area becomes pasture. Land where smallholders' herds graze together is named bathan. Anthrax is said to have been sporadic in bathan areas over many years. In 2009 about 40 animals died with suspected anthrax (all in August–October) and there were 55 human cases [17]. At the same time in 2010 the disease re-emerged in a larger outbreak.

Fig. 1. Geographical distributions of cattle smallholdings of anthrax cases enrolled in the study, Bangladesh, 2010 and the courses of two rivers: Padma and Jamuna in relation to the study area (a), with a closer view of the positions of the case smallholdings and the rivers (b). A bottom/side square box of (b) denotes the number of anthrax case smallholding(s) indicated by the red asterisk. A green shadow encircling a red dot, enclosed in a square box, indicates the magnitude of human cases developed from the case smallholding. The geographical positions of two smallholdings are overlapped. The coordinates of the control smallholdings were not projected because they were from the same area as the case smallholdings.
Case definition, selection of case and control farms
Officially, 44 animals were suspected to have died of anthrax in 2010 in the districts of Pabna and Sirajganj, of which 39 were cattle and four goats (local DLS data). We limited our study to cattle smallholdings. A case smallholding was selected if either of two criteria were met:
-
(1) At least one bovine animal recently died with sudden onset of convulsions or falling down, with or without previous reported fever (seen in the anthrax outbreaks of 2009) [17] and a large number of characteristic organisms (McFadyean reaction) were revealed in the blood films of the moribund or recently dead animals stained with 1% Polychrome Methylene Blue according to the World Organization for Animal Health (OIE) [4]. This was performed at the Field Disease Investigation Laboratory (FDIL) in Sirajganj or at the Central Disease Investigation Laboratory (CDIL) in Dhaka (in Bangladesh one CDIL and eight FDILs provide livestock disease diagnostic services).
-
(2) A cluster of human anthrax cases that had a common history of being exposed in the 3 weeks prior to the slaughter or associated practice(s) following slaughter, of a sick bovine animal belonging to a smallholding. Such a cluster included all the anthrax cases in the community and the smallholder's family, because the sick animal was slaughtered at the homestead of the smallholder to sell meat to neighbours. Slaughter was performed by the Halal method [Reference Gracey, Collins and Huey21]. Practices associated with slaughter included skinning a carcass and processing and cooking meat.
Due to resource constraints organisms stained from the anthrax cases were not further characterized, but according to OIE [4], visualization of McFadyean reaction in blood-stained smears is fully diagnostic for a case of anthrax.
For each case smallholding we selected one control smallholding from the same village. We conducted a census on the numbers of cattle smallholdings which had no clinical ailments and where no antibiotics had been given to the animals during the 3 weeks before the clinical onset of anthrax in the case farm(s). From this sampling frame we selected control farm(s) at random. The incubation period of B. anthracis is up to 14 days [3]. In addition, the owners of control farms were interviewed to ensure that all the animals on the farms were free from febrile diseases over the last 6 months. All case and control farms were selected during July–September, 2010. In a follow-up observation 3 weeks after completion of the questionnaire, no sick animals were found on the control farms.
Data collection and survey methods
To evaluate the risk factors associated with anthrax infection in cattle, data were collected by a questionnaire consisting of 13 binomial variables. The variables included farm management including feeding practice, marked climatic or ecological changes such as heavy rainfall and flooding and presence of arthropod vectors. The variable ‘Some grazing opportunity’ means that the animals grazed only at the homestead; the variable ‘Free-grazing’ means that the animals grazed on any field or pasture. Although there was little chance of finding any difference in the occurrence of heavy rain and flooding in a small geographical area, because of the absence of water-drainage facilities or presence of river canals variation in short-period monsoon downpour or flooding could not entirely be ruled out. Therefore, we also assessed these two variables. The questionnaire was completed by two trained veterinarians during interviews with the farm owners. The farm owners' additional feedback on the probable source(s) of the organism, recorded on a blank space in the questionnaire generated two more variables. Therefore, 15 binomial variables were evaluated which are listed in Table 1. Population statistics of the case and control farms were collected, and Global Positioning System (GPS) coordinates were recorded using personal navigators (eTrex Venture, Garmin, USA). A geographical information system programme (Arc View 9.2; Environmental System Research Institute, USA) was used.
Table 1. Matched-pair analysis of risk factors for anthrax in cattle in Bangladesh (N=15 pairs: 15 case farms, 15 control farms)

OR, Odds ratio; CI, confidence interval; N (+/−), number of exposed case and unexposed control pairs; N (−/+), number of unexposed case and exposed control pairs.
* Matched-pair analysis using McNemar's test.
† Indicates the animals grazed only at the homestead.
‡ Indicates the animals grazed on any field or pasture.
Statistical analysis
The data were entered into a spreadsheet programme (Excel 2000, Microsoft) (the spreadsheet is available on request). To estimate the strength and statistical significance of association between a risk factor and the disease, we applied the matched-pair (McNemar) test using GraphPad Software Quick Calcs (http://www.graphpad.com/quickcalcs/McNemar1.cfm). An association was considered significant if a test had P⩽0·05. The data from the spreadsheet were transferred to Stata v. 9.2 (Stata Corporation, USA) and in order to examine the independence of effects, multivariable conditional logistic regression was applied, using ‘clogit’ syntax. Any variables with P<0·20 after matched-pair analysis were included in the multivariable conditional logistic regression analysis. The model of risk factors was constructed by backward selection applying the iterative maximum-likelihood estimation procedure.
RESULTS
Case and control farms and their population statistics
The locations of the case farms are shown in Figure 1 along with the number of human cases linked to individual case farms. The locations of the control farms are not shown in Figure 1 because the control farms were in the same areas as the cases, and therefore, their locations on the map could be the same. Population statistics of the case and control farms are given in Table 2. The median numbers of cattle observed on the day of survey on the case and control farms were six (range 2–12) and five (range 2–14), respectively. All cattle on the 15 case farms and on 13 control farms were crossbred, but two control farms had Pabna cattle.
Table 2. Population statistics of the case and control farms enrolled in the study for the assessment of risk factors associated with anthrax in cattle in Bangladesh, 2010
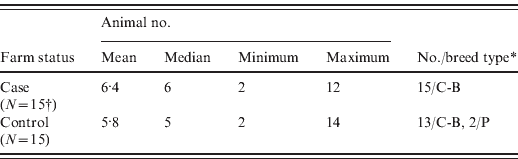
N, Number of farms.
* C-B, Cross-bred; P, indigenous type of cattle named Pabna, after the district [Reference Kamal20].
† Clinical onsets, sequentially: 28/7, 3/8, 10/8, 13/8 (2), 14/8 (2), 15/8 (2), 16/8, 18/8, 21/8, 22/8, 27/8, 28/8.
Risk factors for anthrax infection in cattle
Table 1 shows the results of the matched-pair analysis. The factor ‘Feeding animals with uprooted and unwashed grass’ had the strongest point estimate of effect [odds ratio (OR) 11·0, P=0·009) despite wide confidence interval [95% confidence interval (CI) 1·6–473·5). The only other factor that had P<0·20 was ‘Feeding animals with water hyacinth (Eichhornia crassipes)’ (OR 6·0, 95% CI 0·7–276·0).
Two variables with P<0·20 were considered for inclusion in the multivariable conditional logistic regression analysis to estimate the independence of the effects. After this analysis both remained in the final model as independent risk factors which are ‘Feeding animals with uprooted and unwashed grass’ (OR 41·2, 95% CI 3·7–458·8, P=0·003) and ‘Feeding animals with water hyacinth’ (OR 22·2, 95% CI 1·2–418·7, P=0·039) (Table 3).
Table 3. Final model with potential risk factors for anthrax in cattle on smallholdings in Bangladesh, 2010Footnote *

OR, Odds ratio; CI, confidence interval.
* Conditional logistic regression; final model with two variable entered; χ2 for likelihood ratio test 19·78; P<0·001; pseudo R 2=0·52; no. of observations=30.
DISCUSSION
Because all the 55 human cases of anthrax registered in 2009 were located in the districts of Pabna and Sirajganj [17] and the first human case of the following year was in the same area and at the same time these two districts were selected for study. The sampling frame does not represent the entire country's cattle population, but it is the area in Bangladesh where anthrax occurs.
Herbivores are predominantly infected with anthrax by ingestion of spores [4]. Grass mixed with soil might harbour the spores, and if such grass is offered to an animal it might become infected. Because such feeding is practised in the study area this could be the reason we found the variable ‘Feeding animals with uprooted and unwashed grass’ to be an independent risk factor. Being marooned by water in July–October every year, the farmers in the bathan area mainly practice stall-feeding for their cattle (Table 1). In order to minimize feed cost, they collect any available vegetation for their cattle including water hyacinth and grass roots. Harvesting grass along with roots in an area where a dead animal has been disposed of might contaminate the entire harvest with spores at a lethal level. The germination ability of B. anthracis spores on and around grass roots followed by its vegetative growth has also been reported [Reference Saile and Koehler22].
Locations of farms in a river basin and flooding were suspected of having contributory roles in anthrax outbreaks in North Dakota, USA and in Sweden [Reference Mongoh9, Reference Lewerin12], but to the authors' knowledge ‘Feeding animals with water hyacinth’ has never been reported as a risk factor for anthrax in animals. The clustering in space and time of anthrax cases over the last 2 years may suggest why this floating plant becomes a risk factor for anthrax in the bathan area in Bangladesh. Being located just upstream of the Padma–Jamuna junction (Fig. 1), the grazing becomes submerged during July–October, as mentioned earlier. Due to scarcity of green grass and dry land the farmers frequently feed their cattle with water hyacinth. Farmers also dispose of any dead animals into nearby water [17]. Dumping carcasses in the water may lead to contamination of the water and consequently the water hyacinth.
Four of the case farms had a history of feeding their animals with roots of grass and water hyacinth. Grass roots and water hyacinth are not mixed together, but water hyacinth is sometimes mixed with rice straw before feeding to the animals.
In the bathan area farmers have been feeding their animals with grass-root mixture and water hyacinth for many years. Many factors might contribute to the increased incidence of anthrax in Bangladesh in recent years: one factor might be the catastrophic decline of vultures (Gyps bengalensis, Gyps indicus and Gyps tenuirostris) [Reference Prakash23, Reference Pain24]. The consumption of diclofenac (a non-steroidal anti-inflammatory drug) residues in animal carcasses is a cause of this decline [Reference Oaks25]. Due to the disappearance of vultures, animal carcasses disposed of in fields or water remain exposed for longer, contaminating the soil and water.
Anthrax was reported from two case and three control smallholdings where anthrax vaccine had been used (Table 1). The exact dates of the vaccinations were unknown. Animals vaccinated >6 months ago might not retain sufficient immunity because attenuated Sterne strain vaccine produced in Bangladesh induces immunity that typically lasts for just under 1 year [3]. Annual single anthrax vaccination is recommended by the DLS, but a second dose of vaccine might be considered for an anthrax-prone area [3].
The genotype(s) of the organism that caused animal and human infections in the study period remain unidentified. Multi-locus variable number tandem repeat analysis (MLVA) was performed at the Centers for Disease Control and Prevention (CDC), USA, on the isolates recovered from animals and humans in the 2009 anthrax outbreaks in the same location, which revealed the same genotype [17].
No further anthrax cases were recorded after October 2010 in Bangladesh and because no case diagnosed retrospectively based on only clinical history was included, the sample size of the case farms was small, which could be a reason for not finding more variables as independent risk factors. There was not an option to increase the number of case farms in this study because all the clinically suspected cases in the study area that met the two selection criteria were included. Because no study on risk factors for anthrax in the most vulnerable cattle population in Bangladesh had previously been undertaken, our study generated novel information, which might be helpful in preventing anthrax outbreaks in cattle similar to those seen over the last 2 years.
Animals should not be fed with grass mixed with unwashed grass roots or water hyacinth in the bathan area in Bangladesh, especially during the monsoon and some post-monsoon months (July–October). Storing sufficient rice straw, which is available in the dry season, and cultivation of high yielding green fodder, such as Napier grass, on the homesteads of the smallholders and at public roadsides might be alternative options for safe feed sources during this high-risk period. Because water hyacinth contamination is probably linked to disposal of animal carcasses into water this practice must be stopped. Any dead animal should be disposed of by deep burial on the homestead of the farmer, or on remote dry-land under veterinary supervision for a safer transport of the carcass. Cremation might not be feasible because of cost. Vaccination every 6–9 months for the most vulnerable cattle population might protect animals.
Fifty-five human cases of anthrax were reported in Bangladesh in 2009 and 607 in 2010 [17, 18]. Slaughtering sick animals for meat, handling meat or animal skin or being present at the slaughtering sites were risk factors for human infection [26]. At CDC, USA the same genotype was identified from both bovine and human isolates of the 2009 outbreak [17]. This shows that public heath in Bangladesh is threatened by anthrax in animals at a higher rate in the recent past. Controlling animal anthrax by mitigating the risk factors identified might reduce the number of human anthrax cases in the country.
ACKNOWLEDGEMENTS
Thanks are extended to Dr W. R. Ward, Senior Fellow, University of Liverpool, UK for examining the manuscript. We thank the Director General of DLS, Bangladesh, officials at FDIL, Sirajganj, District and Upazila livestock offices and the owners of the cattle smallholdings for their full cooperation in during the study. This study was funded by Regional Fisheries and Livestock Component (RFLDC), Noakhali, Bangladesh of DANIND by an applied research project grant.
DECLARATION OF INTEREST
None.






