INTRODUCTION
Streptococcus pneumoniae is one of the major bacterial pathogens causing up to 1 million deaths per year in young children worldwide [1]. In the USA about 60 000 cases of invasive disease, including 3300 cases of meningitis are attributed to S. pneumoniae each year [2] and pneumococci are the most common cause of bacterial meningitis in adults [Reference Musher, Mandell, Bennett and Dolin3].
Death and long-term sequelae due to pneumococcal disease are still common features. In the USA the annual number of deaths due to pneumococcal pneumonia and meningitis are estimated to be about 40 000 [Reference Obaro and Adegbola4]. The case-fatality rate for pneumococcal meningitis is reported as high as 30% for developed countries and up to 50% in less developed countries. It is reported that 30–60% of survivors develop long-term sequelae, i.e. neurological deficits and hearing loss [Reference Koedel, Scheld and Pfister5–Reference Auburtin7].
It is acknowledged that most cases of pneumococcal cerebrospinal disease are preceded by bacteraemia, but in rare cases it can be caused by direct extension from otitis media or sinusitis [Reference Musher, Mandell, Bennett and Dolin3, Reference Weber, Tuomanen, Mitchell, Morrison and Spratt8]. Therefore in most cases of meningitis invasion of the respective S. pneumoniae strain from the nasopharynx into the bloodstream is only the first step in pathogenesis. Penetration of the blood–brain barrier is also required. The mechanism of invasion through the epithelium of the nasopharynx is still under investigation. It seems to be dependent on the PspC molecule on the surface of pneumococci and the polymeric immunoglobulin receptor (pIgR) molecule on the surface of respiratory epithelial cells [Reference Hammerschmidt9, Reference Zhang10]. The next step in pneumococcal meningitis, the breach of the blood–brain barrier, seems not to be pIgR-mediated but rather PAF-receptor mediated. The ligand in the cell wall of the pneumococcus seems to be phosphorylcholine [Reference Ring, Weiser and Tuomanen11, Reference Cundell12].
Risk factors for pneumococcal pneumonia and invasive pneumococcal disease (IPD) have been described previously and are part of the latest vaccination recommendations [2, Reference de Roux13–Reference Trampuz16], but to our knowledge no such analysis has been done for pneumococcal meningitis.
Therefore, we conducted a statistical analysis of risk factors for pneumococcal cerebrospinal disease in adults looking at host (age, gender), pathogen (serotype, antibiotic resistance) and environmental (season) factors.
METHODS
Study population
In 2001 a prospective population-based study was started in North-Rhine-Westphalia (NRW). It focused on IPD in adults from NRW. NRW is the most populated federal state in Germany (31 December 2003: 18 079 686 inhabitants, Genesis [17]) and represents almost a quarter of the German population. In 2001, 202 (52%) acute care hospitals and 27 microbiological laboratories serving these hospitals gave consent to participate in this study.
Inclusion criteria were: age of the patient at the onset of disease ⩾16 years, invasive disease confirmed by S. pneumoniae isolation from a normally sterile body site [i.e. blood, cerebrospinal fluid (CSF)], patient living in the area of NRW or being hospitalized in a hospital in NRW at the time of disease. Exclusion criteria were: missing date of birth and/or missing site of isolation of the respective S. pneumoniae strain.
Data
Participating laboratories provided the following information: date of birth, gender, residence of the patient (postal code), material from which the sample was isolated, date of isolation and diagnosis. All isolates were sent to the National Reference Centre for Streptococci (NRCS) where all strains were confirmed to be S. pneumoniae. All pneumococci were serotyped (typing sera from Statens Serum Institut) and minimum inhibitory concentrations (MIC) for penicillin G and macrolides were determined following CLSI guidelines [18]. In cases of missing data we tried to retrieve this by contacting the respective laboratory.
All data were entered into an Access 2000 database.
Statistical analysis
From February 2001 until August 2006, 1174 cases were included in the study. To evaluate the integrity of our electronic data we drew a random sample of 10% (n=120) of our cases and verified relevant study variables with our archived paper records. Apart from one error on ‘gender’, all other data were correct and we thus assumed a high quality of our electronic records.
According to the specimen type cases were assigned as blood (blood culture) or CSF cases. All cases with the diagnosis ‘meningitis’ were assigned as CSF irrespective of the material from which the S. pneumoniae was isolated, i.e. even if the strain sent was isolated from blood. To our knowledge there are no reports on patients with pneumococcal meningitis, where different serotypes of S. pneumoniae were isolated from blood and CSF.
According to the respective date of birth samples were grouped into two age groups: 16–59 years or ⩾60 years. This is because in Germany vaccination against pneumococci is recommended for all people aged ⩾60 years [19].
We used multiple linear regression modelling to examine the adjusted influence of environmental (season), host (age, gender) and pathogen (antibiotic susceptibility, serotype) factors on CSF tropism of S. pneumoniae. Variables were not included in the multivariate model if their P value in univariate analysis was >0·2, as they would contribute very little to model performance.
The parameter season was generated by assigning all samples to one of the following categories: winter (December–February), spring (March–May), summer (June–August) and autumn (September–November). The date of isolation was used for this assignment. In case of missing values we used the date of receipt instead because in our experience strains are normally sent to the NRCS within 10 days of isolation.
MIC values were split into two categories: susceptible and non-susceptible using CLSI breakpoints [penicillin G: susceptible MIC ⩽0·06 mg/l, non-susceptible MIC ⩾0·12 mg/l; macrolides (erythromycin or clarithromycin): susceptible MIC ⩽0·25 mg/l, non-susceptible MIC ⩾0·5 mg/l].
Logistic regressions and 95% confidence intervals (CIs) were calculated using SPSS version 11 (SPSS Inc. USA).
RESULTS AND DISCUSSION
Two thirds of the study population were aged ⩾60 years (Table 1) and there were slightly more samples from men than women (51·8% vs. 47·3%). Ten percent of all isolates came from CSF and 90% from blood. Serotype 14 dominated with 15%, followed by serotypes 3, 7F and 4 with 8·0–8·6% and serotypes 1, 23F, 9V with 5·5–6·3%. Serotypes below 5% were grouped into ‘other’, comprising 42·6% of all samples. In total, 16·5% of all isolates were non-susceptible to macrolides (MIC >0·5 mg/l), and 3·9% of all isolates were not susceptible to penicillin (MIC ⩾0·12 mg/l). Frequency of S. pneumoniae isolation was highest in spring (39·9%), followed by winter (27·3%), summer (16·4%) and autumn (15·1%).
Table 1. Frequency of study variables [absolute and relative (%)]Footnote *
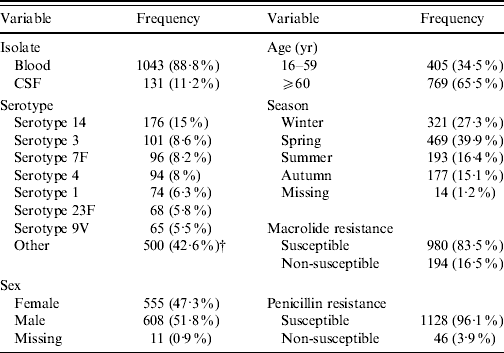
* Absolute number of samples included (n=1174).
† Serotypes 10A, 10B, 11A, 12A, 12B, 12F, 13, 15A, 15B, 15C, 15F, 16F, 17A, 17F, 18A, 18C, 18F, 19A, 19C, 19F, 20, 22F, 23A, 23B, 24F, 25F, 28A, 29, 31, 33A, 33F, 35C, 35F, 36, 37, 38, 5, 6A, 6B, 7C, 8, 9A, 9L, 9N.
Univariate (crude) odds ratios (ORs) showed a statistically significant influence of serotype (P=0·04) and age (P=0·002), whereas sex (P=0·17), season (P=0·92), macrolide resistance (P=0·68) and penicillin resistance (P=0·38) were not significant (Table 2). Season, macrolide resistance and penicillin resistance were not selected for multivariate analysis because of their high P value. Adjusted for each other in multivariate analysis, serotype (P=0·02), age (P<0·001) and sex (P=0·03) were statistically significant. Age (⩾60 years) had an OR of 0·5 (P<0·001) whereas being female had an increased OR of 1·54 (P=0·03). Serotypes 14, 3, 7F and 4 had little impact on CSF ORs (ORs close to 1, all non-significant); however, serotype 1 lowered the OR by 4 (OR 0·24, P=0·03) and serotype 23F increased it by two (OR 2·03, P=0·03). Serotype 9V was non-significant (P=0·11) but showed the tendency to increase the OR for isolation from CSF (OR 1·74).
Table 2. Odds ratios of univariate (crude) and multivariate (adjusted) linear regressionFootnote *
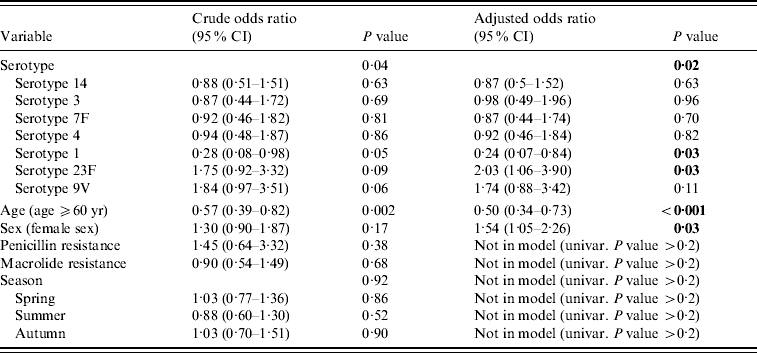
Bold values indicate statistically significant (<0·05) in multivariate linear regression.
* Model: χ2=32·46, d.f.=9, P<0·001, −2 log-likelihood: 777·99 (SPSS version 11).
Age has a well-documented effect on the incidence of IPD [Reference Butler, Tuomanen, Mitchell, Morrison and Spratt20]. One of the two major risk groups for IPD in general are adults aged ⩾60 years. Interestingly, in our study where we wanted to study risk factors for cerebrospinal disease the OR for the variable age is 0·5 (P<0·001), i.e. being aged >60 years ‘protects’ against developing cerebrospinal disease. A possible explanation might be that those living into old age have already encountered a variety of different serotypes earlier in life and developed enough immunity to be protected against CNS invasion in their old age.
Sex as a factor contributing to illness is widely discussed in infectious as well as non-infectious diseases. There are reports on gender differences in parasitic and viral diseases, i.e. in general intensity and prevalence of infection are higher in males [Reference Klein21]. However, in the case of community-acquired pneumonia being female seems to be associated with a higher mortality [Reference Valles22]. In our study being female increased the OR for CNS involvement to 1·54 (P=0·03), i.e. being female is a risk factor for pneumococcal meningitis. This might be explained by the fact that women still spend more time taking care of children [Reference Craig23]. Children have carrier rates of ⩾50% [Reference Bogaert, De Groot and Hermans24] and are a risk population for pneumococcal disease. Therefore women are more likely to be exposed to potentially invasive pneumococcal strains than men and therefore have a higher risk for meningitis, too.
We additionally calculated a model with age×sex interaction to test whether one modifies the effect of the other (data not shown). The interaction term was not significant (P=0·270) and coefficient and P value of the sex variable and the age variable were similar to the model without an interaction term. This strongly supports an effect of age and sex independent of each other.
Season has an influence on the incidence of pneumococcal disease with more cases during wintertime. This coincides with a higher rate of viral infections and it is assumed that cytokines produced during these viral infections facilitate pneumococcal invasion through the epithelium of the nasopharynx by up-regulating receptor molecules [Reference Musher, Mandell, Bennett and Dolin3, Reference Zhang10]. In our study population, about 65% of all infections took place during winter and spring. Nevertheless, season did not significantly contribute to CNS invasion of pneumococci (Table 2).
In recent years rising antibiotic resistance rates in pneumococci were registered and reached alarming levels, e.g. in Spain >50% of isolates were penicillin G resistant [Reference Reinert25]. However, rates for high-level penicillin G resistance in Germany (MIC ⩾2 mg/l) are <2% and rates of non-susceptibility to penicillin are <10% [26]. Therefore penicillin G is still applicable in the initial therapy of IPD in Germany which includes meningitis. Because of its therapeutic value, we calculated an additional model (data not shown) including penicillin resistance into multivariate analysis. However, penicillin resistance was not significant (OR 1·34, 95% CI 0·57–3·16, P=0·506) and adding penicillin resistance as a variable to the age-sex-serotype model did not improve model performance. A log-likelihood test comparing the age-sex-serotype model with the age-sex-serotype-penicillin model was not significant (χ2=0·319, d.f. =1, P=0·572). Finally, we found no evidence that penicillin non-susceptibility has any influence on pneumococcal meningitis in adults in Germany.
In 2005, rates of macrolide resistance among pneumococci in Germany were about 20% in adults and 30% in children [26]. In univariate analysis the influence of macrolide resistance on CNS involvement was minor and statistically not significant (Table 2). Unlike penicillin, macrolides are not a treatment option in IPD and we abstained from including them in multivariate analysis because of statistical as well as clinical reasons.
The capsule of pneumococci is an important virulence factor. It plays a crucial part in the process of colonization and invasion [Reference Bogaert, De Groot and Hermans24]. Furthermore, its capsule protects the pneumococcus from phagocytosis [Reference Jarva27] and elicits a serotype-specific antibody response [Reference Musher28]. There are 91 well-defined serotypes but only 46 were identified in 2005 in IPD in Germany and the ten most common serotypes are responsible for 70% of all IPD in adults [26]. In 2005 it was published that pneumococci induce apoptosis in the endothelial cells of the blood–brain barrier and are thus capable of invading the subarachnoid space [Reference Bermpohl29]. However, these experiments were performed with a serotype 2 strain and not designed to determine a putative role of different capsules in CSF invasion. Nevertheless our statistical analysis indicates that serotypes differ largely in their capability to cause meningitis (Table 2): whereas serotype 1 was inversely correlated to meningitis with an OR of 0·23 (95% CI 0·07–0·84) serotype 23F had an OR of 2·03 (95% CI 1·06–3·90). With everything else being equal, between serotype 1 and serotype 23F there is a greater than eightfold increase in ORs for cerebrospinal infection. At which stage of CSF infection the capsule exerts its influence as yet remains unclear. An earlier report showed that adherence and invasion of encapsulated (opaque) strains of brain microvascular endothelial cells is decreased compared to the adherence and invasion of their non-encapsulated (transparent) isogenic counterparts [Reference Ring, Weiser and Tuomanen11]. But in analogy to pneumococcal septicaemia where only transparent strains colonize the pharynx [Reference Cundell12, Reference Weiser30] and opaque variants are more efficient at establishing bacteraemia [Reference Kim and Weiser31] one might speculate that the capsule exerts its influence during establishment of infection in the CNS. Building on this epidemiological evidence, further studies looking at differences between serotype 1 and serotype 23F might shed more light on the molecular mechanisms of penetration of the blood–brain barrier and subsequent disease.
Finally, with serotype 23F increasing the OR of CNS invasion twofold, we emphasize the importance of high vaccination rates in the general population, as 23F is included in all available pneumococcal vaccine formulations.
ACKNOWLEDGEMENTS
This study was supported in part by funds of Aventis Pasteur MSD, Wyeth and Grant no. RKI415/1369235 of the German Ministry of Health.
DECLARATION OF INTEREST
M.L. has received research project funding from Wyeth Pharma and GSK. M.L. is a member of advisory boards of Wyeth Pharma and GSK. R.R.R. is an employee of Wyeth Vaccines, Paris, France.




