INTRODUCTION
Human campylobacteriosis is the most commonly reported foodborne disease in Switzerland [Reference Schmid1, Reference Wittenbrink2] and the European Union [Reference Westrell3] and has become a leading cause of enteric zoonotic gastrointestinal infections in most developed and many developing countries [4]. In 2007, 6056 cases of campylobacteriosis were reported in Switzerland (83·4/100 000 inhabitants) [5]. Over 90% of these infections are caused by Campylobacter jejuni, about 5% by C. coli and only few by other Campylobacter spp. like C. lari or C. upsaliensis [Reference Wittenbrink2]. The most common symptoms are diarrhoea, abdominal pain, fever, headache, nausea, and vomitus. In most cases, no antibiotic therapy is needed because symptoms are typically self-limiting and last for 3–6 days. Post-infectious complications like reactive arthritis or Guillain–Barré syndrome are rare [Reference Altekruse6].
In Switzerland laboratories are obligated to report human cases of campylobacteriosis to the Federal Office of Public Health (FOPH). Previous studies have suggested that underreporting occurs because notification takes place if a person with campylobacteriosis visits a physician, if the physician takes a stool sample, if the pathogen is detected by a laboratory test and if the positive result is reported [Reference Scallan7]. Therefore the true incidence is higher than that reported and estimates of the number of cases that occur in the community per reported case are important to estimate the total burden and cost of a disease. Estimates exist for certain countries [Reference Wheeler8–Reference Thomas11] but are not available for Switzerland.
The most important risk factors for Campylobacter infection are: handling and consumption of raw or undercooked meat, especially poultry meat; consumption of cross-contaminated ready-to-eat food; travelling abroad; contaminated drinking water; direct contact with infected animals; and consumption of unpasteurized milk [Reference Fullerton12–Reference Rodrigues20]. A case-control study in Switzerland [Reference Schorr21] identified travelling abroad and consumption of chicken liver as the most important risk factors in adults.
To date, most efforts to reduce the risk of campylobacteriosis for humans have focused on poultry and poultry meat [Reference Rosenquist22, Reference Stern23]. In some countries these measures resulted in a considerable reduction in human cases [Reference Stern23, Reference Williman24] whereas in other countries these efforts only had a limited effect in reducing the incidence of campylobacteriosis [Reference Rosenquist22]. One explanation for this may be that other sources of infection such as travel-related infection and imported poultry meat also play an important role. Knowledge on the relative importance of the different sources of human campylobacteriosis is thus important for effective prevention and control measures. Different methods can be used to assess the relative importance of different risk factors of foodborne infections, for example microbiological subtyping, case-control studies, outbreak investigations or exposure assessments [Reference Pires25]. The current study aimed to use existing data on exposure for different potential risk pathways and combine these data in a model that allows integration of information on each pathway. The approach does not require gathering new data and is therefore considered to be cost-effective in estimating the relative importance of different sources.
Data for Switzerland were available for the risk factors poultry meat, travel abroad and pet contact. These risk factors are among the most important factors for sporadic human campylobacteriosis [Reference Fullerton12–Reference Rodrigues20, Reference Pires26]. Other known risk factors were not included either because according to expert opinion these sources are not of major importance in Switzerland (raw milk, contaminated drinking or surface water) or there were not enough data available to estimate the exposure (direct contact with cattle or other animals).
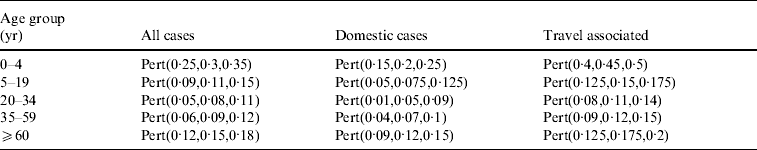
Previous studies suggested that the incidence of campylobacteriosis varies according to age [Reference Schmid1, Reference Studahl and Andersson16, Reference Samuel27]. At the same time, exposure to infection sources such as travel and close contact with pets varies between age groups. This information would be useful for assessing the risk of infection through different sources. A few case-control studies estimated the importance of risk factors for younger children [Reference Fullerton12, Reference Unicomb18], but exposure to infection sources has never been assessed separately for the different age groups.
The objective of this study was to assess the relative importance of three key sources of campylobacteriosis (travelling abroad, poultry meat, pet contact) for different age groups in Switzerland. For this, a stochastic exposure model was used which combined data on Campylobacter incidence for the years 2002–2007 with data on the three exposure pathways, the results of the Swiss case-control study and other studies describing risk factors for campylobacteriosis.
MATERIALS AND METHODS
Outline of the model
The model estimated the incidence of campylobacteriosis in five different age groups over 6 years from available data on exposure to three possible infection sources. A simple infection pathway was modelled for the exposures ‘consumption of poultry meat’, ‘travelling abroad’ and ‘contact with pets’ (Figs 1 and 2).

Fig. 1. Outline of a model designed to attribute Campylobacter cases to infection sources in Switzerland.
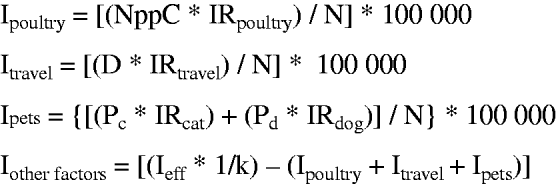
Fig. 2. Mathematical representation of the model where Ipoultry is the Campylobacter incidence due to poultry meat consumption; NppC is the number of Campylobacter-positive poultry meat portions per person per year; IRpoultry is the infection rate for the consumption of a Campylobacter-positive poultry meat portion; Itravel is the incidence due to travelling abroad; D is the number of days spent abroad per person per year; IRtravel is the infection rate for a day spent abroad; Pc is the prevalence of people having daily cat contact; Pd is the prevalence of people having daily dog contact; IRdog is the infection rate for having daily dog contact; IRcat the infection rate for having daily cat contact; Ieff is the incidence of effective reported Campylobacter cases, k is the factor for underreporting and N is the number of people at risk (Swiss population in each age group).
The inputs to the model were data on exposure to each source, the Campylobacter prevalence in the different sources and the estimated infection rate (IR) per exposure, which described the proportion of persons that become ill after a single exposure to a source. This IR was derived from the population attributable fraction (PAF) that was calculated from an odds ratio (OR) for the exposure that had been determined in case-control studies within a specific age group. Based on published ORs and prevalence data and assuming, that for diseases with low incidence the OR is a valid approximation of the relative risk (RR), PAF and IR were calculated as follows [Reference Dohoo, Wayne and Stryhn28]:
where pE+ is the prevalence of Campylobacter-positive exposures within the total number of exposures to the source in the specific age group
where n is the total number of reported cases corrected for underreporting and E+ is the number of exposures to a Campylobacter-positive source within the population of the specific age group.
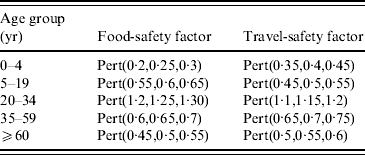
Because it can be assumed that due to different age-dependent risk-mitigating behaviours the IR is not constant across age groups, a correction factor was introduced for the exposure pathways ‘poultry consumption’ (food-safety factor) and ‘travelling abroad’ (travel-safety factor) to adapt the IR to the different age groups.
The output of the simulation model was the incidence of Campylobacter cases/100 000 inhabitants, by year and age group associated with an exposure and the respective PAF.
where E+ is the number of exposures to a Campylobacter-positive source within the population of the specific age group and N the number of persons in this age group.
The incidence of cases attributable to other infection sources which were not included in the model was calculated as the difference between the effective incidence corrected for underreporting and the incidence associated with the three risk factors estimated by the model. If the estimated incidence was greater than the effective incidence corrected for underreporting, the number of cases attributable to other sources was set to zero. A stochastic simulation model was developed using @Risk (Palisade Corp., USA) with 5000 iterations per simulation. (Supplementary material with detailed information on the model is available online.)
Input data
Population and incidence
The population of Switzerland was stratified into five age groups based on demographic data and using reported campylobacteriosis rates from 2002 to 2007 (Table 1). Small children (0–4 years), older children (5–19 years), young adults (20–34 years), adults (35–59 years) and older people (⩾60 years) were grouped together, because it was assumed that behaviour leading to exposure in people within these groups would be similar. Another consideration that was taken into account in defining the age group was the differences in cases reported in the past and published differences in underreporting for these age groups.
Table 1. Data sources used for modelling the exposure

Based on existing estimations of underreporting in other countries [Reference Hall, Raupach and Yohannes10, Reference Thomas11, Reference Adak, Long and O'Brien35], it was assumed that on average 15% of all cases are detected with the current reporting scheme in Switzerland. Based on expert opinion the factor for underreporting was adapted for each age group. In the young children group (0–4 years) 30% of all cases were assumed to be reported, because disease symptoms in this age group are more severe and worried parents seek medical assistance. The lowest reporting rates were assumed for the 20–34 and 35–59 years age groups. To calculate the total effective cases corrected for underreporting the under-reporting factor for ‘all cases’ was used (Table 2). It was also assumed that reporting of domestically acquired infections is half as likely as reporting of infections that are acquired abroad, because people who become ill after travelling abroad were assumed to be more likely to seek medical advice, and stool samples for bacteriological examination would be more likely to be taken. Therefore different factors were used for the calculation of the effective cases associated with domestic exposures and with travelling abroad. Uncertainty on the factor of underreporting was modelled using a Pert distribution (Table 2).
Table 2. Estimated proportion of Campylobacter cases that are reported in different age groups and with different origin of infection (domestic or travel associated) in Switzerland. These proportions were used to correct the reported Campylobacter incidence for underreporting
Poultry meat consumption
The exposure was estimated by calculating the number of Campylobacter-positive poultry meat portions consumed per person per year (Fig. 1). The Campylobacter prevalence in turkey meat is different from that in broiler meat and it is also different in meat of domestic production than in imported meat [Reference Neff30]. Moreover, freezing significantly lowers Campylobacter prevalence. Therefore the consumption per person per year for eight different product categories were estimated (broiler or turkey meat/fresh or frozen/import or domestic production).
The quantity of poultry meat consumed per person per year is not the same in different age groups. It was assumed that this difference was mostly due to different portion sizes and not to a different number of portions. Therefore it was concluded that the average number of portions in each age group is more or less the same and could be estimated by taking an average portion size of 150 g across all age groups.
The prevalence of C. jejuni and C. coli on poultry meat was estimated from data on the Campylobacter prevalence on broilers in the slaughterhouse (years 2002–2006) and on broiler meat at retail (2007). The prevalence of Campylobacter on turkey meat was estimated from a prevalence study at retail in 2005 [Reference Neff30]. The apparent prevalence (AP) was corrected with the test specificity (Sp) and test sensitivity (Se) to estimate the true prevalence (TP) using the following formula [Reference Dohoo, Wayne and Stryhn28]:
The IR was estimated based on the OR for poultry meat consumption from a Swiss case-control study [Reference Schorr21]. With the OR the PAF for the 20–34 and 35–59 years age groups was calculated and based on this the IR per portion of consumed Campylobacter-positive poultry meat for these age groups was derived. The minimum, mean, and maximum values of IR for poultry consumption calculated for the years 2002–2007 were used to define the Pert distribution reflecting the uncertainty of a general IR for poultry consumption.
Various studies reported that especially young adults (19–34 years) are at higher risk of Campylobacter infection probably because of inappropriate kitchen hygiene [Reference Regula31, Reference Nauta, van der Fels-Klerx and Havelaar36], which should result in different IR for poultry meat consumption in different age groups. In our model, the estimation of the IR was based on data of the young and middle-aged adults (19–34 and 35–59 years, respectively). Therefore this rate had to be adapted with a food-safety factor for the different age groups which was based on expert opinion on estimated kitchen hygiene in each group. The 0–4 years age group was considered to have the lowest risk (i.e. high kitchen hygiene) with the 20–34 years age group having the highest risk which was reflected in the applied food-safety factors (Table 3). Uncertainty of the values of the food-safety factor was modelled using a Pert distribution.
Table 3. Factors used to correct the estimated Campylobacter infection rates for poultry consumption (‘food safety’) and travelling abroad (‘travel safety’). Different infection rates were used in different age groups to correct for differences in risk behaviour
The number of cases associated with poultry consumption was calculated by multiplying the number of consumed infected portions per age group with the IR corrected with the food-safety factor for each age group and the number of persons in this age group.
Travelling abroad
Exposure was estimated by assessing the number of days spent abroad per year for each age group (Fig. 1). Statistics on days spent abroad were obtained from data of the Swiss Federal Statistical Office (SFSO) and data published by the Institute of Public Services and Tourism of the University of St Gallen (Table 1). The trips were stratified into short (<4 overnight stays) and long (⩾4 overnight stays) trips. Based on the proportions of travel abroad for short and long trips and the mean duration of a short and a long trip in the different age groups, the total days spent abroad were calculated for each year.
The OR for travelling abroad from a Swiss case-control study [Reference Schorr21] was used to derive the IR per day abroad for the 20–34 and 35–59 years age groups. The prevalence of travel exposure for these age groups was calculated by dividing the days spent abroad by the total days per year in these age groups. The minimum, mean, and maximum values of the IRs for travelling abroad calculated for the years 2002–2007 were used to define a Pert distribution as an input for the IR per day abroad used in the model.
It was assumed that IR per day abroad was not the same in different age groups, because young adults especially were assumed to follow hygienic recommendations for travellers less strictly than persons from other age groups. In our model, the estimation of the IR was based on data of the young and middle-aged adults (19–34 and 35–59 years, respectively). Therefore this rate had to be adapted with a travel-safety factor for the different age groups according to expert opinion. Uncertainty of the values of the travel-safety factor was modelled using a Pert distribution (Table 3). The number of cases associated with travelling abroad was calculated by multiplying the number of days spent abroad in this age group with the IR corrected with the travel-safety factor for this age group.
Contact with pets
Exposure was estimated by assessing the prevalence of persons in each age group with daily contact with an infected cat or dog (Fig. 1). Data on the penetration of dogs and cats in households of different categories (young families with children, young families with teenagers, adult families, young couples without children, older couples without children, young singles, older singles) were obtained from a pet-nutrition company, which conducts a yearly telephone survey on this matter. By estimating the percentage of persons in the different age groups belonging to the given household categories, the number of persons with a pet in the same household was calculated (daily dog/cat contact).
Prevalence data of C. jejuni and C. coli in cats and dogs, corrected for the test sensitivity and specificity, were used to calculate the number of persons with daily contact with an infected pet [Reference Wieland34].
To estimate the IR for a daily contact with a pet, the OR data for cat and dog contact of a Swedish case-control study [Reference Studahl and Andersson16] were used. The IR for daily cat/dog contact for the years 2002–2007 were calculated and the minimum, mean, and maximum values of this output were used to define a Pert distribution to derive the average IR for daily cat/dog contact.
It was assumed that IR was the same in all age groups. The number of cases associated with daily cat/dog contact was calculated by multiplying the number of persons with daily cat/dog contact with the IR for daily cat/dog contact.
Other risk factors
The incidence due to risk factors not included in the model was calculated by subtracting the estimated incidences due to poultry consumption, travelling abroad and pet contact from the total incidence corrected for underreporting (Fig. 2). To estimate the total reported cases, the estimated reported cases associated with poultry consumption, travelling abroad and pet contact were subtracted from the total effective reported cases and the means of these differences over the years for every age group were calculated. For each age group these values were defined as model outputs and the minimum (truncated at 0), mean, and maximum of these outputs were used to define a Pert distribution for the mean of the reported cases associated with other risk factors.
Sensitivity analysis
The effect of uncertainty and variability of input variables on the model outputs ‘incidence associated with poultry consumption’, ‘incidence associated with travelling abroad’, ‘incidence associated with pet contact’ and ‘total incidence’ was assessed using the sensitivity analysis tool of the @Risk software. With multivariate stepwise regression the standardized β coefficients for the associated input variables of an output were calculated. The most influential variables were determined by the magnitude of the standardized β coefficients.
RESULTS
Exposure to risk factors
Campylobacter prevalence of broiler meat decreased from 53·8% (95% CI 49·8–58·2) in 2002 to 27·5% (95% CI 25·4–29·7) in 2006 and then increased again in 2007 to 52% (95% CI 41·2–63·7).
Poultry consumption also decreased from 9·7 kg per capita per year to 8·2 kg per capita per year in 2006 and increased again in 2007 to 9·5 kg.
Therefore the number of estimated consumed Campylobacter-positive portions per person per year halved from 31·2 (95% CI 25·0–39·4) in 2002 to 14·2 (95% CI 11·5–18·3) in 2006 and then increased again to 29·3 (95% CI 22·9–37·6) in 2007 (Fig. 3).

Fig. 3. Number of Campylobacter-positive poultry meat portions consumed per person per year (–◆–), days spent abroad per person per year (- -▪- -) and percent of population with daily cat contact (![]() ) or dog contact (
) or dog contact (![]() ) for the years 2002–2007 in Switzerland.
) for the years 2002–2007 in Switzerland.
The number of days spent abroad steadily increased from 2002 to 2007 in all age groups (Fig. 3). Small children spent the fewest and young adults (20–34 years) the most days abroad. In small children the number of days spent abroad increased from 6·5 (95% CI 5·8–7·0) to 9·5 (95% CI 8·7–10·4) and in young adults from 13·9 (95% CI 12·6–15·3) to 23·1 (95% CI 20·8–25·4), respectively.
The prevalence of daily cat/dog contact remained constant in all age groups (Fig. 3) and was highest in children aged 5–19 years with 33% of the people in this age group having daily cat contact and 17·4% having daily dog contact. The prevalence of daily cat/dog contact was least in older people, with 22·7% having daily cat contact and 14% having daily dog contact.
Model outputs
The model outputs for PAF for the different exposure pathways in the different age groups from 2002 to 2007 are shown in Table 4. Travelling abroad and poultry consumption were both responsible for 19% (mean values cited), respectively, of the Campylobacter cases in small children. Ten percent of the cases could be associated with direct pet contact and 51% were due to other risk factors.
Table 4. Population attributable fractions for Campylobacter infection due to poultry consumption, travelling abroad, pet contact and other risk factors in five different age groups in Switzerland for the years 2002–2007

M, Mean; CI, confidence interval.
In the older children group (5–19 years) 29% of the cases were attributable to poultry consumption. The percentage of cases due to travelling abroad in this age group was 23%. Nine percent of the cases were associated with pet contact and 39% with other risk factors.
In the adult age groups (20–34 and 35–59 years) PAF for poultry consumption was 24% and 25%, respectively. The percentage of cases associated with travelling abroad was 32% in both age groups. Pet contact was associated with 3% of the cases in the 20–34 years age group and 6% in the 35–59 years age group. The percentage of cases that could be attributed to other risk factors was 42% for the 20–34 years age group and 37% for the 35–59 years age group.
In elderly people PAF for poultry consumption was 36%. Twenty-nine percent of the cases were associated with travelling abroad and 10% with pet contact. In this age group 25% of the cases could be attributed to other risk factors.
Mean PAF values over all age groups and years were 27% (95% CI 17–39) for poultry consumption, 27% (95% CI 22–32%) for travelling abroad, 8% (95% CI 6–9) for pet contact and 39% (95% CI 25–50%) for other risk factors (Fig. 4).

Fig. 4. Population attributable fraction (mean over the years 2002–2007) for pet contact (▪), travelling abroad (□), poultry consumption (![]() ) and other risk factors (
) and other risk factors (![]() ) in five age groups in Switzerland.
) in five age groups in Switzerland.
From 2002 to 2007 incidence due to travel abroad increased from 807·4 to 1211·7 cases/100 000 inhabitants. Incidence associated with poultry consumption first decreased from 1271·3 to 588·7 cases/100 000 in 2006 and then increased again to 1994·1 in 2007. Incidence due to pets remained stable with 211·9 cases in 2002 and 219·9/100 000 in 2007, whereas incidence due to other factors constantly decreased from 2013·2 to 1093·9/100 000 inhabitants.
As seen in Figure 5 the highest mean Campylobacter incidence after correcting for underreporting was found in the 20–34 years age group with a mean incidence of 1512·2 cases/100 000 inhabitants per year. In small children, the mean estimated incidence was 372·9 cases/100 000 inhabitants per year.

Fig. 5. Estimated incidence of Campylobacter cases corrected for underreporting (mean over the years 2002–2007) due to pet contact (▪), travelling abroad (□), poultry consumption (![]() ) and other risk factors (
) and other risk factors (![]() ) in five age groups in Switzerland.
) in five age groups in Switzerland.
Sensitivity analyses
The uncorrected IR per portion of poultry meat and the portion size had the biggest influence on the model outcome ‘incidence associated with poultry consumption’, with β coefficients in the different age groups ranging from 0·61 to 0·72 and −0·53 to −0·64, respectively. The correction factor for poultry consumption and the test sensitivity were also significantly influencing this outcome with β coefficients ranging from −0·14 to −0·21 and 0·24 to 0·34, respectively. The influence of Campylobacter prevalence in broiler meat varied between meat categories with β coefficients from 0·17 to 0·24 for prevalence on frozen broiler meat and 0·06 to 0·15 for prevalence on chilled broiler meat.
The uncorrected estimated IR per day abroad had the greatest influence on incidence of Campylobacter cases associated with travelling abroad with β coefficients ranging from 0·88 to 0·92, followed by the mean duration of travels with 4–7 overnight stays (β coefficients 0·24–0·4) and the different correction factors for age groups (β coefficients 0·1–0·28). In adult age groups (20–34 and 35–59 years) the mean duration of travels with 31–60 overnight stays had a major influence on the incidence associated with travelling with β coefficients of 0·23 and 0·34, respectively.
The model outcome ‘incidence associated with pet contact’ was mostly influenced by the IR for daily dog/cat contact with β coefficients ranging from 0·61 to 0·83.
The total reported incidence was mostly influenced by the uncorrected IR per portion of poultry meat (β coefficient 0·44), the mean estimated reported cases associated with other risk factors for age groups 20–34 years (β coefficient 0·39) and 35–59 years (β coefficient 0·38), the uncorrected IR per day abroad (β coefficient 0·36), the portion size (β coefficient −0·26) and the mean estimated reported cases associated with other risk factors for age groups ⩾60 years (β coefficient 0·23) and 5–19 years (β coefficient 0·18). Other influential factors include the factors for underreporting in age groups 20–34 years (β coefficient 0·17) and 35–39 years (β coefficient 0·14), and test sensitivity (β coefficient −0·1).
DISCUSSION
Based on source-attribution modelling, the relative importance of three key exposure pathways for human campylobacteriosis in different age groups was estimated over a period of 6 years.
The outputs of the model indicate that there are considerable differences between age groups. This should be taken into account in future information campaigns depending on the target audience. The age group where the fewest cases could be attributed to any of the risk factors explored were small children. There are several case-control studies that show that the risk factors for small children are different from those for adults [Reference Fullerton12, Reference Tenkate and Stafford17, Reference Unicomb18]. Moreover, a genotyping study conducted in Scotland [Reference Strachan37] showed that small children in rural regions had a higher risk for campylobacteriosis than those in urban regions and that young children in urban areas were more likely to be infected with poultry-associated Campylobacter strains, whereas children in rural regions were more likely to be infected with ruminant-associated strains. In Switzerland there are no specific studies on risk factors for small children and more research is needed to address this important knowledge gap.
Over all age groups and over all years of our analysis, a mean proportion of 27% (95% CI 17–39) of cases were attributed to poultry consumption indicating that this source of infection is still of major importance in Switzerland. This is in accord with the results of several case-control studies from other countries that found proportions of cases associated with the consumption of chicken meat ranging from 23·8 to 29·3% [Reference Friedman13, Reference Wingstrand15, Reference Stafford38, Reference Doorduyn39]. Recent source attribution studies based on the multilocus sequence typing method (MLST) from England, Scotland and New Zealand estimated that 50–80% of human cases were related to the chicken reservoir [Reference Strachan37, Reference Wilson40–Reference Mullner42]. This difference can, on the one hand, be explained by the fact that part of the cases that are due to travel abroad are associated with poultry consumption in a foreign country and with genotyping methods would be associated with poultry. On the other hand some of the MLST studies exclude cases associated with travel, which results directly in a higher percentage of cases associated with poultry. Additionally, strains from the poultry reservoir may infect humans not only by the food pathway but also by the environment or by direct contact. These pathways were not considered in our model. A comparison between poultry meat source attribution based on case-control and genotyping studies suggested that differences could be due to an overestimation by genotyping because of yet incomplete data on other reservoirs than farm animals and also due to an underestimation by case-control studies because of misclassification due to immunity, resulting in exposed people not becoming ill [43].
The output of our model indicated that if the Campylobacter prevalence on poultry meat could be lowered to zero, 27% of the human Campylobacter cases could potentially be prevented. Campylobacter spp. is widespread in the environment and a common component of the avian gut flora. It is therefore very difficult to prevent chickens from becoming colonized and even stringent biosecurity measures cannot predictably keep campylobacters out of a poultry flock [44, Reference Rosenquist, Boysen and Bork45]. Therefore, only a reduction of the level of contamination may be achievable and this will only be possible if stringent safety measures are applied throughout the whole food-production chain.
Our finding of 27% (95% CI 22–32) of cases attributable to travel appears to be high compared to estimates in other studies [Reference Fullerton12–Reference Neimann14] but is in accord with results of a recent case-control study from the UK, where 24% of cases travelled abroad in the previous 14 days [Reference Tam46], and a meta-analysis of 37 case-control studies on risk factors for campylobacteriosis, that found international travel to be the most important risk factor for sporadic campylobacteriosis [Reference Pires25]. The importance of this risk factor in a population is not only dependent on travel activities and duration but also on preferences in travel destinations. The risk for Campylobacter infection is higher for travel to Asia and Africa than for travel within Europe [Reference Ekdahl and Andersson47] and the relative importance of this pathway is also dependent on the proportion of cases that are infected in the country of residence. Our model did not take travel destination into account because there were no data available on the association between travel destination and the risk of campylobacteriosis infection for Switzerland. The estimated importance of travel for elderly people is high, with 29% of cases associated with this source of infection. This could be due to the fact that in Switzerland elderly people travel frequently, with a proportion of 82–89% having at least one trip with at least one overnight stay [Reference Laesser and Bieger33]. Our results suggest that comprehensive information about hygienic behaviour for travellers is still of major importance for the prevention of Campylobacter cases in Switzerland.
Our model estimated that 10% of the Campylobacter cases in small children and in elderly people are due to daily pet contact, whereas in young and middle-aged adults only 3% and 6%, respectively, were attributed to this infection pathway. These findings are in agreement with other studies that estimated 2·9–7% of the cases were due to frequent contact with dogs or cats [Reference Stafford38, Reference Doorduyn39]. There are no known interventions that could lower the prevalence of Campylobacter in pets, therefore the only measure to lower the risk from this source is to promote hygiene in contact with animals.
Recent source attribution studies based on microbial subtyping suggested that ruminants, especially cattle and sheep, were a major source for human campylobacteriosis [Reference Strachan37, Reference Wilson40–Reference Mullner42]. This source was not included here because a genotyping study from Switzerland suggested that isolates likely to originate from cattle accounted for <10% [Reference Wieland48]. Meat from ruminants and raw milk cheese is generally considered to be of low risk [49, Reference Bachmann and Spahr50] and raw milk is only rarely consumed in Switzerland. However, occupational and environmental exposure pathways for ruminants might be relevant and should be investigated.
Figure 6 shows a comparison of the total reported cases per year estimated with the model and the effective reported cases per year in Switzerland. For 2002 the model underestimated the cases, whereas for the years 2005–2007 the cases were slightly overestimated. Regarding the relative importance of sources over time, our results suggest that the decrease in reported Campylobacter cases from 2002 to 2006 was mainly due to a decrease in exposure by poultry meat. Campylobacter prevalence on broiler meat and poultry consumption decreased in this time period and then increased again in 2007. This finding again highlights the importance of this exposure pathway.

Fig. 6. Comparison of the reported Campylobacter cases in Switzerland (–◆–) and the estimated reported cases using the risk attribution model (![]() ) for the years 2002–2007.
) for the years 2002–2007.
The amount of cases due to other risk factors differs from year to year, with a decrease in four of the five age groups. This risk factor covers the residual proportion of cases that could not be attributed to other sources. It is therefore sensitive to changes in the other risk pathways. Changes between years could be due to one or several of the following reasons. The input variable ‘Campylobacter prevalence in broiler meat’ is based on data from different sources. Different sampling strategies or different laboratory methods could have influenced the apparent prevalence in different ways. Due to lack of information these differences could not be accounted for in the model. Another possible explanation for the different amounts of cases due to other risk factors is an effective change in the exposure to other sources. Multiple sources with changing Campylobacter prevalence over the years, for example surface water or other animal and food sources, could be responsible for these differences.
One limitation of the model is that it did not take immunity into account while epidemiological evidence suggests that infection with Campylobacter can induce protective immunity, not only in highly exposed humans in developing countries but also in lesser exposed individuals in industrialized countries [Reference Havelaar51, Reference Janssen52]. During Campylobacter infection innate, cellular and humoral responses are induced but the role of these mechanisms in conferring immunity is yet unclear. Immunity appears to prevent illness but not colonization [Reference Janssen52]. However, recent serosurveillance suggested that the majority of Campylobacter infections are asymptomatic [Reference Havelaar51]. The decreasing amount of cases due to other risk factors with age could therefore be explained with an increase in immunity. As young children are not protected, they could become ill when they are exposed to sources which harbour low Campylobacter doses, whereas elderly people only get ill when they are exposed to sources with relatively high Campylobacter doses. High prevalence in young adults could be explained by high exposure via travel and poultry pathways, where probably the highest doses of Campylobacter can be found. However, more knowledge on immunity to Campylobacter in different age groups is needed for the development of more realistic models that incorporate host susceptibility factors.
The advantage of the model presented here is the introduction of the IR per exposure, which allows combination and comparison of different dimensions of exposure (IR per portion of poultry meat, per day spent abroad, daily dog/cat contact).
As highlighted with the results of the sensitivity analysis, uncertainty of the model is mostly due to the estimation of the IR per exposure. The estimation of the IR for poultry consumption and travelling abroad were based on the results of the only one case-control study available for Switzerland with data from the year 1991 [Reference Schorr21]. It is possible that more than 10 years later these data may have limited validity. The prevalence of Campylobacter in poultry meat in 1991 and therefore the exposure to Campylobacter through poultry consumption is unknown. Travelling behaviour, especially the choice of destinations is likely to have changed over the years which could result in a different OR for travelling abroad. However, the high OR for travelling abroad appears realistic when considering the findings of a study on the molecular epidemiology in Switzerland where a large proportion of human isolates did not show any similarity with isolates found in Swiss poultry, cattle or pets [Reference Pires25, Reference Wieland48], and thus could be related with infection abroad. IR for daily pet contact was based on the results of a case-control-study from Sweden, and given the cultural similarities of the two countries this approach can be justified. To obtain better estimates of the IRs, a case-control study which includes all age groups is necessary. This would also help to further elucidate the differences found in age groups and adjust the correction factors used in our model. Nevertheless, compared to data of more recent case-control studies from other countries the OR data used for our model still seem to be realistic [Reference Fullerton12, Reference Friedman13, Reference Wingstrand15, Reference Unicomb18, Reference Danis19, Reference Stafford38].
One data gap identified was in relation to underreporting of enteric diseases, and reporting behaviour in different age groups. No data were available on this for Switzerland, and we used results from studies performed abroad [Reference Wheeler8, Reference Kuusi9, Reference Thomas11, Reference Adak, Long and O'Brien35]. Reporting behaviour needs to be better understood in order to develop accurate epidemiological assessments, not only for campylobacteriosis, but also for other diseases.
One strength of the model presented here is its flexible structure which allows the introduction of more exposure pathways when data become available. Exposure sources to consider would include ruminants and direct or indirect contact with other potential carrier animals and contaminated food as well as recreational activities, in particular if associated with surface water. There is a considerable proportion of Campylobacter cases that cannot be explained by any of the sources considered, which indicates that more research on risk factors is needed in Switzerland. Nevertheless the findings of our study can be used to inform future attempts to prevent campylobacteriosis and that risk communication strategies should account for differences in age groups.
NOTE
Supplementary material accompanies this paper on the Journal's website (http://journals.cambridge.org/hyg).
ACKNOWLEDGEMENTS
We thank Bell AG, Zell (Dr F. Renggli and Ch. Schatzmann), Mars Schweiz AG, Switzerland (Dr J. Henner), Prodega/Growa Cash+Carry (A. Schärz), Moosseedorf and Proviande (M. Schneider), Bern for kindly providing prevalence and consumption data, and Dr J. Danuser for his input in the early stages of the project.
DECLARATION OF INTEREST
None.












