INTRODUCTION
Estimates of the burden of foodborne disease have been undertaken in a number of countries [Reference Mead1–Reference Hall3]. These estimates rely on multiple steps and data sources but in essence the first step is to conduct an epidemiological study to estimate the total burden of syndromic gastroenteritis – which can be due to various oral transmission routes including food, water, environment or person-to-person – and the second step is to attribute a proportion to foodborne transmission. The estimation of the proportion of gastroenteritis that is foodborne disease has depended on information on known pathogens that are ingested by the oral route. Importantly, the methods have not always taken account of the possibility that acute gastrointestinal symptoms may be due to infections that are acquired through a primary respiratory route rather than an oral route. This could potentially lead to inflation of the final estimate of the burden of illness that is foodborne.
There is evidence that some respiratory infections may cause gastrointestinal symptoms such as vomiting and diarrhoea, and some gastrointestinal infections may cause respiratory symptoms, such as sore throat, nasal discharge, sneezing, and coughing [Reference Monto and Koopman4–Reference Rahman7]. However, it is often difficult to distinguish between respiratory infections with concomitant gastrointestinal symptoms, gastrointestinal infections with concomitant respiratory symptoms, and co-infections. This presents a problem when gastrointestinal symptoms are used to define enteric infections, such as in estimates of foodborne diseases [Reference Mead1–Reference Hall3, Reference Lake8]. The inclusion of persons who have gastrointestinal symptoms but do not have an enteric infection (e.g. a person with an infection of respiratory origin and gastrointestinal symptoms, but without enteric infection) will inflate the estimate of enteric infections. When estimating the burden of foodborne illness in the USA, Mead et al. tried to account for this by adjusting the incidence of gastroenteritis downwards by the proportion of persons with gastrointestinal symptoms that was probably due to respiratory infections [Reference Mead1]. In Australia, Hall et al. excluded some persons with gastroenteritis who also reported concomitant respiratory symptoms [Reference Hall3]. Population-based studies have been used to estimate the incidence of acute gastroenteritis in a number of countries [Reference Flint9]. However, the role of respiratory symptoms as exclusion criteria for the case definition of acute gastroenteritis has not been examined closely.
The objectives of this study were threefold: (1) to describe the frequency of respiratory symptoms in persons with acute gastrointestinal symptoms; (2) to determine the factors associated with respiratory symptoms in persons who also reported gastrointestinal symptoms and (3) to demonstrate the potential impact on estimation of the burden of acute gastroenteritis, when persons with respiratory symptoms are excluded from the case definition of ‘acute gastroenteritis’. To achieve these aims, we examined data from population-based surveys in Australia, Canada, and the USA with comparable study designs, time-frames, and with common variables including those related to respiratory symptoms.
MATERIALS AND METHODS
Data
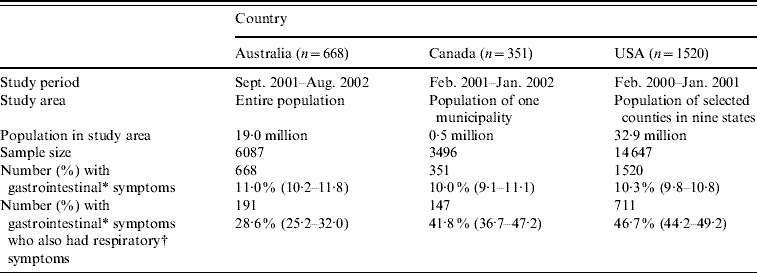
Population-based surveys of gastroenteritis were conducted in Australia, Canada and the USA between 2000 and 2002. All three surveys were conducted over a 12-month period and employed a similar telephone methodology, summarized in Table 1 and described in detail elsewhere [Reference Imhoff10–Reference Hall12]. Sample sizes varied by country.
Table 1. Study methodology, sample size, and number of respondents with gastrointestinal and respiratory symptoms for the Australian, Canadian and USA data used in this analysis
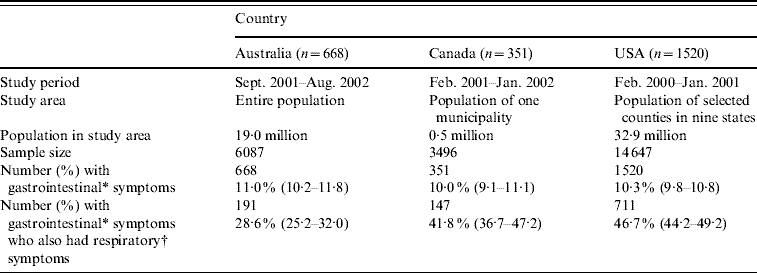
Values in parentheses are 95% confidence intervals.
* Gastrointestinal symptoms defined as diarrhoea, vomiting or both in the 4 weeks prior to the interview, excluding those who reported chronic illness as the likely cause (unweighted).
† Respiratory symptoms defined as at least one of sore throat/nasal discharge, sneezing, or coughing during the gastrointestinal episode (unweighted).
A case of acute gastroenteritis was defined as a respondent who had experienced diarrhoea, vomiting, or both, in the 4 weeks prior to interview. Diarrhoea was defined as ⩾3 loose stools in a 24-h period. Respondents with diarrhoea, vomiting, or both, were excluded from further analysis if their gastrointestinal symptoms had been previously diagnosed as relating to non-infectious causes such as chronic illnesses, pregnancy or excessive alcohol consumption. Cases were asked about the occurrence of respiratory symptoms as part of their illness including sore throat, nasal discharge, sneezing, or coughing. Information was also collected in the survey on other symptoms, demographic characteristics, and healthcare-seeking behaviours.
Analysis
The proportion of cases with acute gastroenteritis that had respiratory symptoms was calculated for each country. Of persons with acute gastroenteritis, factors associated with respiratory symptoms were evaluated in each country using univariable and multivariable logistic regression with presence or absence of respiratory symptoms as the outcome. The dichotomized explanatory variables included sex, household income (above or below $50 000 per annum in each country's currency), antibiotic usage in the 4 weeks before symptom onset, blood in stool, cramps, fever, headache, vomiting, visiting a doctor, and submitting a stool sample for laboratory testing. Categorical variables included age groups (<5 years, 5–24 years, ⩾25 years), season (winter, spring, summer, autumn), duration of vomiting, diarrhoea, or both (1, 2–3, ⩾3 days), and number of loose stools (0, 1–2, ⩾3 stools per 24 h). The months included in each season differed between the two hemispheres, as appropriate. As the studies from Canada and Australia had smaller samples we opted to be inclusive of possible associations and defined statistical significance as P<0·1.
Multivariable analysis used a backwards-stepwise process with forward re-introduction (P<0·1 for backwards elimination, and P<0·2 for re-introduction). The effect of regional stratified sampling in the Australian survey was accounted for by including state in the model. There was no clustering in the study design in any country. Weighting was not included in the logistic regression since the purpose of the analysis was to identify relative odds ratios (OR) in risk factors. Variables found to be significant in backwards multivariable analysis in at least one country were included in a final comparative model, run for each country with all the variables held in the model. Biologically plausible two-way interactions were examined in these comparative models. As longer duration may increase the probability of having a second infection at the same time, the constellation of symptoms associated with respiratory symptoms was examined for cases with short (⩽3 days) and long (>3 days) duration of acute gastroenteritis separately to see if there was a difference in the pattern of symptoms.
The effect of excluding cases with concomitant respiratory symptoms from a case definition of acute gastroenteritis was examined. Estimates of the incidence of acute gastroenteritis were compared first, when the case definition excluded those with any respiratory symptom and second, when the case definition excluded those who had respiratory symptoms plus fever and headache. Estimates were weighted for population age and sex. Analysis was performed using the statistical packages SPSS version 15 [13], SAS version 9.1 [14] and Stata version 8.0 [15].
RESULTS
Of 6087 respondents in the Australian survey, 668 (11%) had acute gastroenteritis, and of these, 191 (29%) reported concomitant respiratory symptoms. Of 3496 Canadian respondents, 351 (10%) reported acute gastroenteritis, and of these, 147 (42%) reported concomitant respiratory symptoms. Of 14 647 respondents in the USA, 1520 (10%) reported acute gastroenteritis, of which 711 (47%) reported concomitant respiratory symptoms.
Univariable analysis
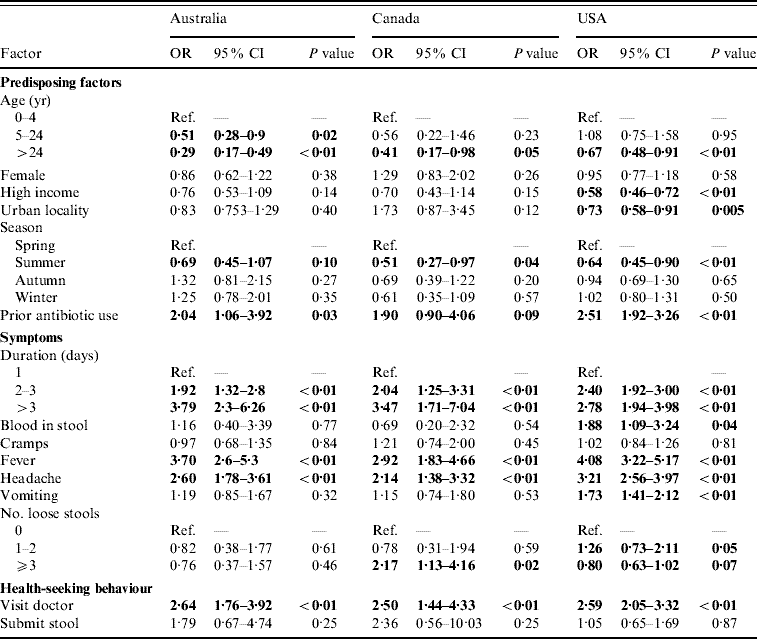
For persons with acute gastroenteritis, the factors associated with having respiratory symptoms were similar across countries (Table 2). Persons aged ⩾5 years were less likely to have respiratory symptoms than children aged <5 years. Reporting respiratory symptoms was least likely in the summer months. Persons who took an antibiotic in the 4 weeks or month before onset of diarrhoea were more likely to have concomitant respiratory symptoms.
Table 2. Factors associated with respiratory symptoms in cases of acute gastroenteritis in Australia, Canada, and the USA in univariable analyses
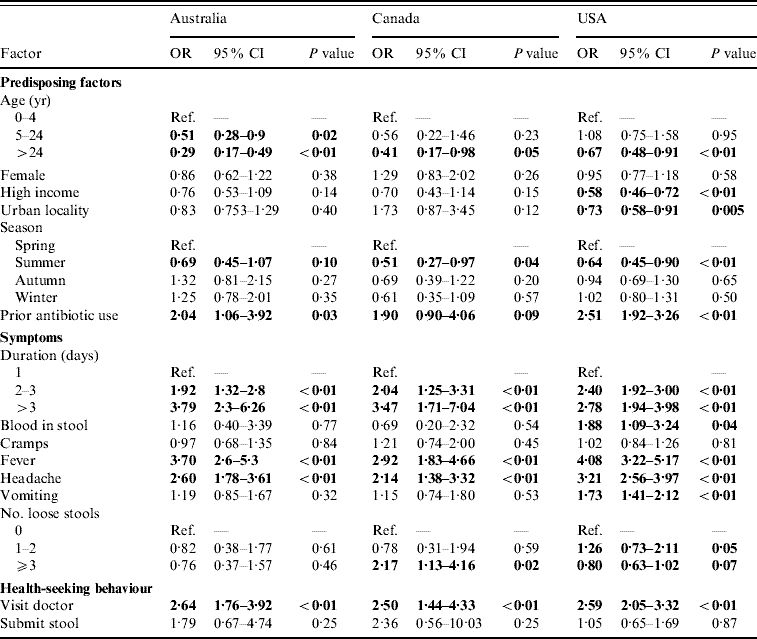
OR, Odds ratio; CI, confidence interval.
OR significant at P=0·1 are shown in bold face.
Persons with a duration of diarrhoea, vomiting, or both >1 day were more likely to have respiratory symptoms than persons with duration ⩽1 day; this association became stronger as the duration of illness increased. Persons with headache and fever were more likely to have respiratory symptoms than persons without headache and fever. Visiting a physician was associated with respiratory symptoms but submitting a stool specimen was not.
Multivariable analysis
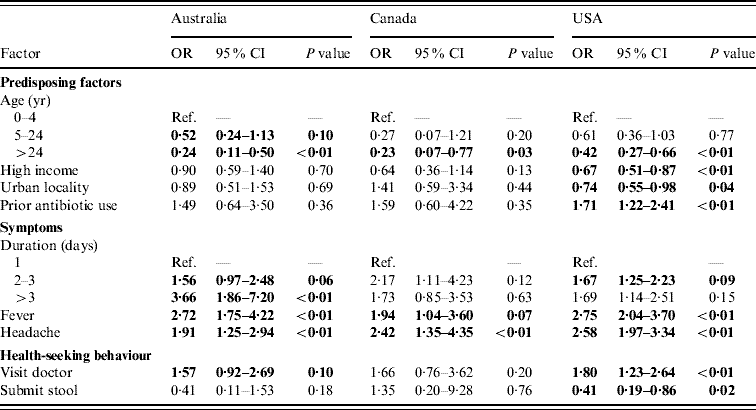
Of persons with acute gastroenteritis, factors associated with respiratory symptoms in multivariable regressions in at least one country were age <5 years, income >$50 000, living in an urban area, prior antibiotic use, duration of diarrhoea, vomiting, or both ⩾3 days, having fever, having headache, visiting a doctor for the illness, and submitting a stool sample. These variables were included in the final comparative models (Table 3) and the direction and magnitudes of the ORs were generally similar across countries, although statistical significance was more often reached in the USA where the sample size was larger. Of persons with acute gastroenteritis, having a fever, and having a headache were statistically significantly associated with having respiratory symptoms in all three countries. Increased duration of diarrhoea, vomiting, or both, had statistically significant increased odds of respiratory symptoms in the USA and Australia. Age ⩾5 years was associated with lower odds of having respiratory symptoms in all three countries. Prior antibiotic use, visiting a doctor, and income <$50 000 were positively associated with respiratory symptoms in all three countries but were only statistically significant in the USA. In persons with acute gastroenteritis, submitting a stool sample was statistically significantly less likely if respiratory symptoms were present in the USA.
Table 3. Factors associated with respiratory symptoms in cases of acute gastroenteritis in Australia, Canada, and the USA in multivariable analyses
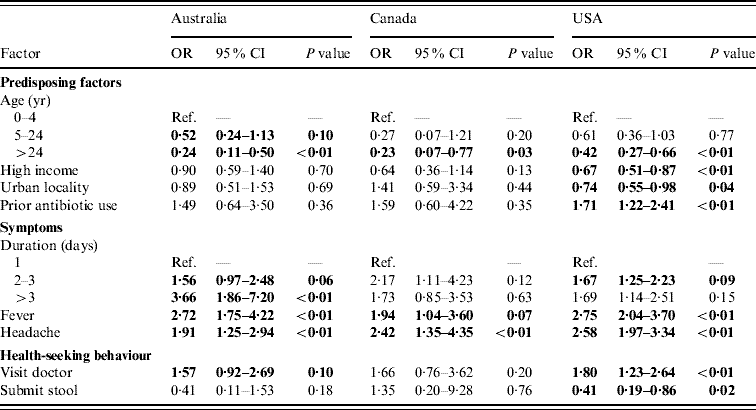
OR, Odds ratio; CI, confidence interval.
Odds ratios significant at P=0·1 are shown in bold face.
The same multivariable analysis was repeated separately for cases with duration of diarrhoea, vomiting, or both; ⩽3 days and >3 days. In persons with acute gastroenteritis, fever and headache remained statistically significantly associated with respiratory symptoms in cases of both long and short duration.
Estimates of incidence of acute gastroenteritis when excluding cases with respiratory symptoms
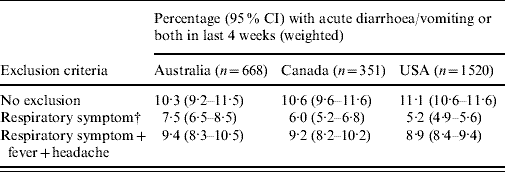
The changes in the weighted estimates of the incidence of acute gastroenteritis when excluding persons who reported respiratory symptoms are shown in Table 4. Compared with the estimates with no respiratory exclusion criteria, excluding persons with respiratory symptoms resulted in a decrease in the estimate of acute gastroenteritis of >50% in the USA (5·2% compared to 11·1%), and decreases of over 40% and 30% in Canada and Australia, respectively. Excluding persons with respiratory symptoms plus fever and headache resulted in decreases of 10–20% compared to the estimates with no respiratory exclusions.
Table 4. Variation in estimates of acute gastroenteritisFootnote * when excluding cases with respiratory and other symptoms in Australia, Canada and the USA
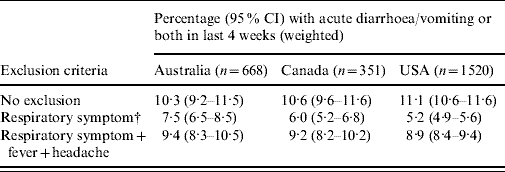
* Gastroenteritis defined as diarrhoea, vomiting or both in the 4 weeks prior to the interview, excluding those who reported chronic illness as the likely cause.
† Respiratory symptoms defined as at least one of sore throat/nasal discharge, sneezing, or coughing during the gastrointestinal episode.
DISCUSSION
In this study we found that respiratory symptoms occurred frequently in persons with acute gastrointestinal symptoms (diarrhoea, vomiting, or both) in population-based studies in Australia, Canada and the USA. In addition, there was a tendency for a common constellation of symptoms to be associated with respiratory symptoms, especially headache and fever which were observed in all three countries. Adjustment of the case definition of acute gastroenteritis to remove cases with respiratory symptoms can cause a considerable decrease in the estimates of the incidence of acute gastroenteritis.
In developed countries, acute gastroenteritis has previously been reported more in women, children, and persons with higher education which is consistent with the data in the studies presented here [Reference Monto and Koopman4, Reference Majowicz11, Reference Hall12, Reference Herickstad16–Reference Papaello18]. Respiratory symptoms have been reported more frequently in those living in lower-income households [Reference Cohen19], under increased stress [Reference Cohen, Tyrrell and Smith20], children [Reference Monto and Koopman4, Reference Imhoff10, Reference Meijer21] and adults with young children [Reference Jaakkola and Heinonen22]. In our study, we found that in persons with acute gastroenteritis, children aged <5 years were more likely to have concomitant respiratory symptoms. Persons with acute gastroenteritis with low income in the USA were also statistically significantly more likely to have concomitant respiratory symptoms. The higher rate of concurrent respiratory symptoms in these groups could be due to a differential in the types of pathogens more frequently encountered in different socioeconomic groups.
The average incidence of respiratory infections, irrespective of acute gastroenteritis, is estimated to be between 2 and 3 per year in Australia [Reference Leder23]. Upper respiratory tract infection is characterized by runny nose, cough, sore throat, fever, headache and an illness that lasts an average of 5 days [Reference Leder23]. The incidence of acute gastroenteritis (excluding chronic causes) in Australia, is close to one episode per year with a mean duration of 2 days [Reference Hall24]. The probability of onset of a respiratory illness on any given day is therefore about 3/365 or about 0·0082. Given that someone has a gastrointestinal infection lasting 2 days, and assuming independence of infections, the probability of having a respiratory infection of 6 days that overlaps the 2 days of gastroenteritis can be estimated as 1 – (1−0·0082)7 or 0·06. Even allowing for non-independence of infections, it remains improbable that all the cases with vomiting and/or diarrhoea with respiratory symptoms in our study can be explained by also having a concomitant, separate respiratory infection.
Earlier studies in the USA and Australia found the prevalence of respiratory symptoms in persons with confirmed gastroenteritis to be 27% [Reference Monto and Koopman4] and 35% (M. Sinclair, personal communication), respectively. These results are similar to the prevalence we observed in the population survey in Australia (29%), but lower than that observed in Canada (42%) and the USA (47%). In cases with confirmed respiratory organisms in the USA, gastrointestinal symptoms were noted in 11% of respiratory cases [Reference Monto and Koopman4]. Conversely, in persons with laboratory-confirmed enteric infections in Australia, a range of prevalence of concomitant respiratory symptoms was reported depending on the enteric organism identified [Reference Sinclair25]. Respiratory symptoms were found in 21% of Campylobacter cases, 24% of patients with norovirus infections, 44% with Salmonella sp. infections and 81% with rotavirus infections. It seems likely that a proportion of enteric infections causing acute gastroenteritis probably do have respiratory symptoms as part of the illness, perhaps especially for rotavirus infections. However, it also seems likely that a proportion of those with a primary respiratory illness also have symptoms of diarrhoea, vomiting or both. It is also possible that some cases of diarrhoea/vomiting with respiratory symptoms could have an underlying chronic respiratory condition such as hay fever or rhinitis, although the wording in the survey questionnaire enquired about symptoms in the current illness and was more likely to elicit responses about acute infection rather than chronic symptoms. It is interesting that in the studies in the USA and Australia, respiratory symptoms were associated with a reduced likelihood of submitting a stool sample suggesting that physicians may regard these cases as respiratory infections and do not consider it appropriate to order a test.
This paper raises a basic question about the appropriateness of using syndromic population-survey data to estimate the burden of foodborne illness, although even expensive cohort studies with a laboratory component cannot be relied on to identify all cases that are respiratory in origin. A large proportion of symptomatic cases remain with an unidentified cause of their illness even after samples are routinely sent to the laboratory. However, further work with laboratory-confirmed gastrointestinal and respiratory infections in cohort studies may be informative about the likelihood of occurrence. Our overall conclusion is that at least some acute gastroenteritis cases with respiratory symptoms may be due to respiratory infections. This strongly indicates to us the need to carefully consider this issue when estimating the burden of foodborne disease based on data from population studies. It is important to first clearly describe the burden of diarrhoeal disease and possible syndromic overlaps in the population as part of the estimation process. The results of this study indicate that considerable over-inflation of estimates of the burden and cost of foodborne diseases may occur if consideration is not given to cases possibly resulting from respiratory infections. In the past, a few studies have dealt with this issue in several ways [Reference Mead1, Reference Monto and Koopman4, Reference Hall12] but further work is required to fully elucidate this issue. In the interim, future population surveys of symptom-based case definitions for acute gastroenteritis could collect data on respiratory symptoms. This will allow consideration to be given to adjustment for cases showing respiratory symptoms.
ACKNOWLEDGEMENTS
The authors thank the OzFoodNet Working Group in Australia, the Emerging Infectious Program FoodNet Working Group in the USA and the National Studies on Acute Gastrointestinal Illness (NSAGI) initiative collaborators in Canada (in particular, Susanna Ogunnaike-Cooke, for data analysis and review of the manuscript). Funding for these studies was provided by the respective governments of Australia, Canada and the USA.
DECLARATION OF INTEREST
None.