INTRODUCTION
Over the last two decades, livestock test and slaughter management, coupled with targeted control of wildlife, has proved successful in reducing the prevalence of bovine tuberculosis (bTB) in New Zealand domestic cattle and deer herds. For example, the number of infected cattle herds fell by 94% from a peak of 1484 in 1994 to just 91 by mid-2010 [1]. Nevertheless, infection of wildlife with Mycobacterium bovis (the causative agent of bTB) persists in many regions, with the principal reservoir and maintenance host being the introduced brushtail possum (Trichosurus vulpecula) [Reference Coleman and Cook2]. By 2007, it was reported that wildlife control operations had reduced possum populations on almost all farmland within the areas most affected by bTB, but there remained over 1 million ha of mostly forested unfarmed habitat in which infected possum populations were not yet under control [1]. In the absence of control, regional possum population densities can reach extremely high levels, and in favourable habitats such as broadleaf forest, up to 20 animals/ha have been recorded [Reference Efford and Montague3].
It has been estimated that without control there would be around 50 million free-ranging possums in New Zealand, but this has been reduced to nearer 30 million [4], mostly as the result of intensive aerial poison-control operations and ground-based poisoning/trapping operations. While the incidence of M. bovis infection in possums in bTB-endemic areas varies, and is usually low (<2%), rates of up to 60% infection have occasionally been recorded [Reference Coleman5]. Infection with M. bovis is also common in three other introduced wildlife species in New Zealand: ferrets (Mustela furo), feral pigs (Sus scrofa) and wild deer (mostly red deer Cervus elaphus). However, none of these are generally regarded as maintenance hosts at the low densities at which they mostly occur in the wild in New Zealand [Reference Coleman and Cook2, Reference Nugent6]. Nevertheless, all three can act as temporal and/or spatial vectors of infection, capable of transmitting M. bovis infection and possibly creating new outbreaks of infection in possums or livestock [Reference Corner7]. Here we have investigated whether, and to what degree, a reservoir of M. bovis infection in feral pigs might persist once the source of infection from possums is reduced or eliminated.
Pigs in New Zealand are susceptible to M. bovis infection, and generally develop fibrotic or mineralized tuberculous lesions in the lymph nodes of the head region [Reference Lugton8, Reference Wakelin and Churchman9]; it has been suggested that oral exposure during scavenging probably represents the primary route of infection in wild pigs in this country [Reference Coleman and Cook2, Reference Nugent6–Reference Wakelin and Churchman9]. Tuberculous lesions are rarely, if ever, fulminating and pigs have long been regarded in New Zealand as probable end hosts for the pathogen [Reference Morris and Pfeiffer10]. An end-host/non-transmitting status of feral pigs has been previously deduced from studies in Northern Australia: there, bTB disappeared from feral pigs when infected populations of cattle and buffalo (Bubalus bubalis) were first eliminated, even though the pigs themselves were not controlled [Reference McInerney, Small and Caley11, Reference Radunz12]. However, in New Zealand, the prevalence of M. bovis infection in adult pigs can sometimes approach 100% among feral populations, even though the prevalence of infection in possums may be only a few percent [Reference Coleman, Caley and Montague13]. Hence, farmers and disease managers find it difficult to discount pigs as contributors to the ongoing persistence of infection in wildlife, as do some European researchers [Reference Machackova14]. Adding to that uncertainty, overseas studies in Spain, focusing on regions with no cattle and few wild deer, have indicated an overlap in M. bovis strain types between wild boar and free-ranging semi-domestic Iberian pigs, suggesting that transmission between pigs – in the absence of a second host species – can occur [Reference Serraino15–Reference Vicente18]; further, bTB infection has been recorded in wild boar on Corsica, despite the apparent absence of co-infection of other wild mammals there [Reference Richomme19].
Here, we investigated the potential for feral pigs to act as a temporal reservoir and vector of M. bovis in New Zealand. We conducted a series of large-scale field experiments aimed at determining whether bTB could be maintained independently among pigs, in a region where bTB is known to be prevalent in possums, if population control is applied to the latter species. By reducing or eliminating the source of infection from bTB-infected possums, we sought to determine whether possum control alone was sufficient to eliminate bTB from wildlife. We compared trends in the age-specific prevalence of M. bovis infection in pigs in six areas, three in which the possum populations were controlled, and three in which they were not. We also characterized the likely spread of disease by infected pigs, and explored the implications of that for bTB management in New Zealand.
MATERIALS AND METHODS
Design
bTB prevalence data on pigs for this study were assembled from three separate projects; (i) a 1996–2003 investigation of bTB in wild deer and (incidentally) feral pigs; (ii) a 2004–2006 investigation of bTB in sympatric cattle, possums, ferrets, and feral pigs; (iii) a 2003–2007 investigation of bTB in feral pigs designed specifically to address the aims above. The main dataset comprised cross-sectional necropsy data from feral pig surveys of six areas, with repeated surveys of each of the areas spanning 4–11 years. Possum populations were controlled in three of the areas but not in the other three. Pre- and post-control possum abundance was estimated (indexed) using the nationally standardized trap-catch index (TCI) method [20]. The TCI is the percentage of leg-hold traps set for one night in which a possum is captured. We were unable to comprehensively survey possum numbers to determine absolute abundance during the study, but varying amounts of TCI data were available for each area, either from related projects or from performance monitoring routinely undertaken by management agencies, to verify the effectiveness of possum control. Previous research and herd-testing data indicated bTB had been present in wildlife in all six areas for at least a decade before this study began (see below).
Study areas
The three non-treatment areas (i.e. no possum control applied) were:
(i) Omoto Forest (OF; ~28 000 ha; 42° 32′ S, 171° 18′ E). Pigs were surveyed in 2002, 2004, and 2007. Despite not being controlled, possum densities in the vicinity were moderate or low (0·1–1·8 possums/ha overall, but >5/ha in localized patches [Reference Coleman and Fraser21]), but bTB was widespread in deer and possums [Reference Nugent6] and bTB prevalence in possums was high (19%) in some places [Reference Coleman and Fraser21].
(ii) Dillon Cone, Molesworth Station (DC; ~17 500 ha; 42° 16′ S, 173° 13′ E). Pigs were surveyed in 2004 and 2006. In early 2005, in a separate study, we recorded a TCI of 13% over ~30 000 ha that included DC. Based on the TCI–density relationship documented by Ramsey et al. [Reference Ramsey22] this indicates a possum density of about 2–3 possums/ha. The prevalence of bTB in the possums captured was 2·0% (G. Nugent and J. Whitford 2008, Landcare Research unpublished report LC0708/032).
(iii) Muzzle Station (MS; ~6500 ha; 42° 09′ S, 173° 31′ E). This area is southeast of the DC area above, with similar terrain and habitat. Pigs were surveyed in 2002, 2004, and 2007, and additionally two TB-infected pigs were obtained in 2003. A digital terrain model, integrating habitat and possum-trapping data, predicts somewhat higher overall possum abundance than in the nearby DC area above (A. Byrom, Landcare Research, unpublished data) with a TCI of 10–40% over about half of the study site.
The three treatment areas were:
(i) Bullen's Hills area of Molesworth Station (BH; ~8700 ha; 42° 23′ S, 173° 07′ E). Pigs were surveyed in 2004 and 2006. This area lies immediately west of the of the DC area above, again with similar habitat and possum density (i.e. 2–3 possums/ha). We necropsied 192 possums collected from this area in spring 2004 and confirmed bTB in 1% of them (G. Nugent and J. Whitford 2008, Landcare Research unpublished report LC0708/032). Following that survey, BH and its surrounding area were aerially poisoned in October 2004. No post-operation TCI data were available for the BH site itself; however, two adjacent areas were poisoned 4 years later using an identical operation and these reduced possum abundance by >90% to <2% TCI (I. Yockney and G. Nugent 2008, Landcare Research unpublished report LC0809/065); the assumption was therefore that the 2004 poison operation at BH would have been of similar success.
(ii) Waitohi Gorge (WG; ~25 000 ha; 42° 54′ S, 172° 25′ E). Pigs were surveyed in 2002, 2004, 2005, and 2007. A possum TCI of 19% was recorded in part of the area in 2004 [Reference Coleman, Coleman and Warburton23], but no bTB prevalence data are available for possums. Ground-based control of possums using traps and poisons was initiated in peripheral areas in 2003 but full control was not achieved until 2004, reducing possum abundance to <1%TCI (P. Spencer, Environment Canterbury, unpublished data).
(iii) Eastern Hauhungaroa Range (EH; ~25 000 ha, 38° 44′ S, 175° 36′ E). Pigs were surveyed in most years between 1995 and 2007. A 2% bTB prevalence was recorded in possums on the eastern edges of the area in the early 1980s [Reference Pfeiffer24]. A possum TCI of 21% was recorded in part of the area in 1993 [Reference Nugent25]. The area was aerially poisoned in 1994–1995, 2000–2001, and 2005. Possums were also controlled on the farmland to the east of the area throughout this period, but possums in the contiguous area to the west were not controlled at all until 2001, and not completely successfully until an exceptionally intensive control operation conducted in 2005 reduced possum abundance to a TCI of 0·04% [Reference Nugent25, Reference Coleman, Fraser and Nugent26]. bTB was last recorded in possums within the EH area in 1995, but was still present in the area to the west in early 2005 (J. Coleman and G. de Lisle 2007, Landcare Research unpublished report LC0607/106).
The OF and EH areas consist largely of unfarmed forest while the WG, MS, DC, and BH sites are mostly unforested rough farmland. The latter four sites are all mountainous, with high ridges separating isolated strips of pig habitat in the valley bottoms, whereas in the other areas pig habitat was more contiguous.
Capture and necropsy of pigs
Pigs were killed by direct observation and shooting, and the carcasses provided for necropsy. Most of the samples were obtained by recreational hunters who usually provided just the heads of the pigs for survey; however, some pigs were shot by research staff or contractors in the course of other work and in these cases the whole pig carcass was necropsied. In total, necropsy data were obtained from 785 pigs; these comprised 274 whole-carcass necropsies with the remainder comprising all head region nodes plus various intestinal tract nodes, where available. The sex, location, and date of death were recorded for each pig. Necropsies were conducted either in the field or at purpose-designed facilities, and involved visual inspection and then, where practicable, the removal and thin slicing of the following groups of tissues and nodes to be examined for lesions:
(i) Head: submaxillary, parotid, retropharyngeal, and atlantal lymph nodes, and the oropharyngeal tonsils.
(ii) Thoracic cavity (where available): the pleura and lungs plus the bronchial, apical, and mediastinal lymph nodes.
(iii) Abdominal cavity (where available): the liver, kidney, the hepatic and renal lymph nodes, the ileocaecal and ileojejunal lymph nodes associated with the intestines, and the internal iliac lymph nodes.
(iv) Peripheral lymph nodes (where available); the inguinal, popliteal, precrural and prescapular nodes.
Because the head region lymphatic system is the most frequent site of M. bovis infection in pigs in New Zealand [Reference Lugton8, Reference Wakelin and Churchman9], tissues pooled from the submaxillary lymph nodes of both sides of the head were submitted from all pigs for bacteriological culture identification of M. bovis infection (National Centre of Infectious Disease Laboratory, AgResearch, Wallaceville), including those with no visible lesions (NVL). In addition, material from any tissues with lesions or pathology considered typically (TYP) or atypically but possibly (EQUIV) indicative of bTB were cultured separately, but usually with no more than 2–3 cultures submitted per animal.
The age (in months) of each pig was determined from the dentition of the lower jaw [Reference Clarke27] and assigned to an age group (0–1, 1–2, 2–3, >3 years). The latest possible year of birth was estimated by subtracting the age at death from the date of death.
Statistical analyses
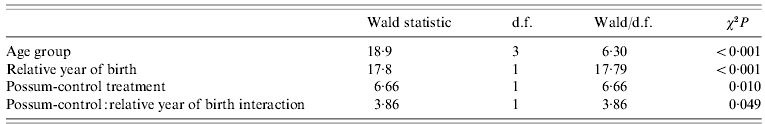
The 95% confidence intervals (CIs) about estimates of bTB prevalence in pigs were calculated according to Collet [Reference Collet28]. The effect of possum control on bTB prevalence was estimated using a mixed-effect, generalized linear model (GLM) with binomial errors. The independent fixed-effect variables fitted were treatment (the presence or absence of successful possum control), birth year, sex, age (age group), with area also included as a random effect. For this analysis, the earlier 2000/2001 and 1994/1995 control operations in the EH area were excluded, since they had not provided the expected level of possum control [based on unpublished trapping data indicating the continued presence of high densities of possums immediately west of the study area until 2005, and the presence of bTB in that possum population until 2005 (J. Coleman, unpublished data)]; however, data following the intensive poison operation of 2005 at the EH site were included.
Initially the four fixed-effect variables and all of their two-way interactions were fitted using the GLM procedure in the statistical package R (R Development Core Team, 2008). Non-significant terms (P>0·05) were sequentially dropped until a minimum adequate model remained (i.e. one with only statistically significant terms).
To examine the spatial distribution of infection in pigs in one of the areas, i.e. EH, a GLM with binomial errors was used to model bTB status, with period (1994–2000 and 2001–2004), sex, age group, and distance from the western boundary as predictors.
RESULTS
Patterns of infection
Across the six study areas, tissue samples from 785 pigs were assessed for the presence of tuberculous lesions and bTB infection. Culture-confirmed infection with M. bovis was recorded in 293/785 pigs sampled, representing an overall infection prevalence of 37·3% (95% CI 34·0–40·7). In the culture-confirmed cases, 95% of animals presented with TB-like lesions or pathology suggestive of mycobacterial disease in the lymph nodes of the head region, indicating only a low incidence of non-clinical or early-stage infection. Sex had not been recorded in 26 of the 785 cases, but where it had been recorded infection rates were marginally higher in females (40·2%, 95% CI 35·6–45·0, n=410) than males (34·1%, 95% CI 29·3–39·2, n=349). The prevalence of M. bovis infection varied with age (see below) and between areas and years (Table 1). Some of this variability is likely to have reflected wide variation in age structure and area-wide representativeness as a result of small sample sizes.
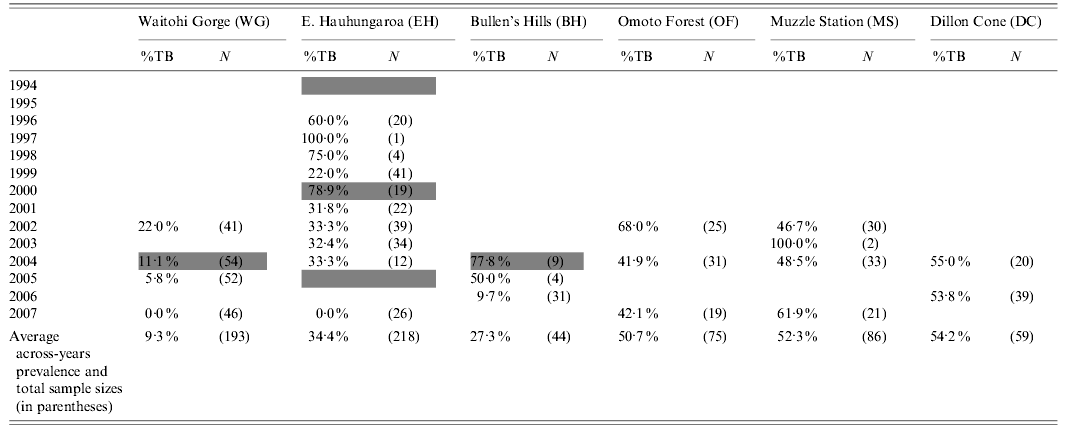
Table 1. Prevalence of culture-confirmed M. bovis infection cases (%) and sample sizes (in parentheses) of pigs from six main areas, by year of survey. In addition, pigs were obtained in 2004 from an area immediately south of WG (WGS; 3·4% prevalence, n=29) and for the Lake McRae area immediately east of DC (2004/2005, 94·5% prevalence, n=56; 2006; 76·0% prevalence, n=25) and these additional 110 cases were included in the overall prevalence analysis. The shaded cells indicate the years in which possum control was applied within study areas
To confirm previous observations that bTB in wild pigs in New Zealand is most often identified by M. bovis lesions in head-region lymphatics [Reference Lugton8, Reference Wakelin and Churchman9], a subset study of whole-carcass necropsies (n=50, from 274 available carcasses) was undertaken to verify this phenomenon. Overall, 37 out of these 50 pigs had suspect TB-like lesions in the head-region lymphatics and 36 of these samples were culture-confirmed with M. bovis infection; in contrast, only 5/50 animals had extracephalic TB-like lesions (all in the ileocaecal lymph nodes), and all five of these cases co-presented with head-region lesions which were culture-confirmed for M. bovis, hence indicating that whole-carcass necropsies for the entire set of 785 pigs sampled would have been unlikely to have increased diagnostic sensitivity for disease or infection status.
Effect of possum control on M. bovis infection in pigs
For all three non-treatment areas, the prevalence of bTB in sampled pigs remained moderate or high in the final surveys in each area (Table 1). In contrast, it was low or zero in the areas in which possum control had been successfully implemented.
Many of the pigs killed after possum control had been born before the control was implemented, so were exposed to both pre- and post-control forces of infection. This tended to obscure the effect of control. That effect is shown more clearly by the trends in prevalence in relation to birth year (Fig. 1). In the three areas in which possums were not controlled, there was either no evidence of declining prevalence in successive cohorts, or the decline was minor. In contrast, bTB prevalence in pigs fell substantially in all three areas with possum control.
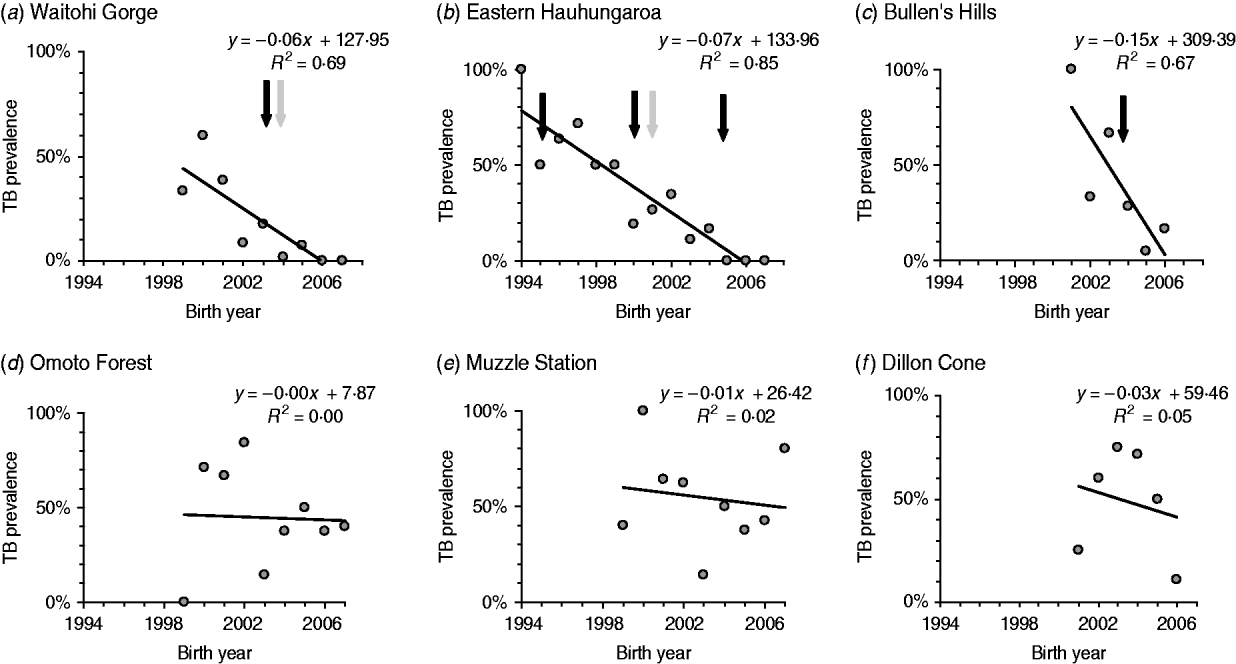
Fig. 1. bTB prevalence in pigs in relation to birth year. Simple trend lines are fitted to the observed prevalence for each cohort in each area, with each point treated equally regardless of the differences in sample size between cohorts. The last three cohorts in each sequence have age structures that are increasingly biased towards the young age groups [e.g. the 2004 cohort for panels (b–d), and the 2005 cohort for panel (a) contain only the youngest (0–1 year) pigs]. Possum control was applied to areas in panels (a–c) but not in areas in panels (d–f). The dark arrows indicate when possums within the area were controlled and the lighter arrows indicate when possum control was applied to adjacent areas.
The minimum adequate model for bTB prevalence indicated clear fixed effects only for age group, birth year, and possum control, with a weak interaction between birth year and possum control (Table 2), in line with age-specific prevalence data above and the patterns evident in Fig. 1. The prevalence of infection in pigs born after possum control (in the areas controlled) was lower (3·6%, 95% CI 1·5–8·2, n=137) than in pigs sampled at the same time that had been born before control (12·5%, 95% CI 6·9–21·5, n=80). The difference is significant (Fisher's exact test, P=0·02), and implies that most of pigs had become infected before possum control. The significant effect of birth year indicates that M. bovis infection prevalence in pigs also declined over time in the areas without possum control, although to a lesser degree than in the areas with control (Fig. 1).
Table 2. Statistical significance of fixed-effect terms in the minimum adequate generalized mixed-effects model of factors affecting the bTB infection status of feral pigs in the six main study areas. Only data from 2000 onwards were included, and relative year of birth represents the year of birth expressed in relation to the year in which full possum control was first achieved in those areas that received control, and in relation to the midpoint of the study for those area where possums were not controlled
Further inspection of Fig. 1 indicates a pre-2005 decline in bTB prevalence among pigs in EH that was progressive rather than immediate (between 1994 and 2005 possums were progressively controlled by only partially successful poison operations, but not brought under full population control at the whole-landscape scale until the intensive poison operation of 2005). Given the abrupt effect of possum control on bTB prevalence in pigs at the other two sites, the more drawn-out decline in infection prevalence in pigs at the EH site is consistent with progressive improvement in control coverage over a longer period of time.
Collectively, the results indicate that when possum populations were successfully reduced to low levels at whole-landscapes scales, the prevalence of bTB in pigs dropped to near zero levels within 2–3 years.
Spatial pattern of infection
The progressive nature of poison operations in the EH area represents a spatial pattern of possum control; this facilitated more detailed investigation of spatial variation in bTB prevalence in relation to changing possum relative abundance. As already noted, the EH area sensu lato was the subject of possum control operations in 1994/1995 and again in 2000/2001, and the adjacent areas to the north, east and south were deemed to have been brought under control progressively; however, an area immediately to the west was not brought under full control until the intensive operation of 2005. In that western area, M. bovis infection was common in deer, pigs, and possums until at least 2000 [Reference Nugent6], and in May 2005 was confirmed by mycobacterial culture as still present at 11% prevalence in possums (n=93) in a moderate-high density population collected from a small (~200 ha) part of the adjacent western area ([Reference de Lisle, Yates and Coleman29] and J. Coleman, Landcare Research, unpublished data).
M. bovis infection prevalence in pigs killed inside the EH area remained high after the initial control operation in 1994, with an average for the 1996–2000 samples of 47% (95% CI 36·8–57·7, n=85). After the second round of possum control in 2000/2001 control, it fell to 32·7% (95% CI 24·5–42·1, n=107) for the 2001–2004 samples, and then quickly to zero when the possum populations on both sides of the range were reduced to near zero by intensive poisoning in 2005 (TCI=0·04%, n=15 000 trap nights [Reference Pfeiffer24]).
Coupled with the evidence above from other areas, which indicates that landscape-scale possum control quickly reduced bTB prevalence in pigs to near-zero levels, these result indicate that between 1996 and 2005 pigs were mostly becoming infected to the west of the EH study area and then ranging into EH and being killed there. In line with that, there was a strong east–west gradient in bTB prevalence in the EH area (Fig. 2), and bTB-infected pigs aged <1 year were on average killed closer to the western boundary than were older TB-positive pigs. The minimum adequate model included three significant factors: age (<1 and ⩾1 year), period (1994–2000 or 2001–2005), and distance from the western boundary, with no significant interactions (Fig. 2).
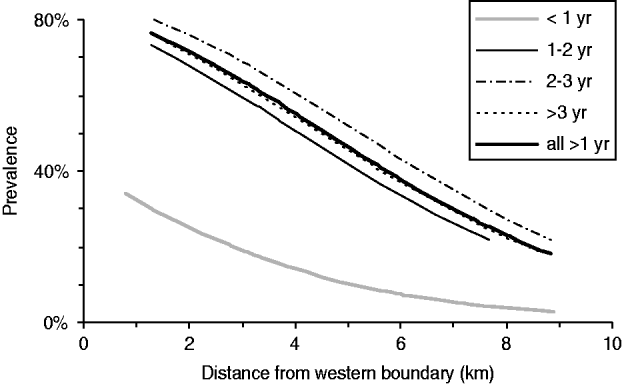
Fig. 2. West–East gradient in the prevalence of bTB in pigs in the East Hauhungaroa region during the 2001–2005 period, for each age group, and for all pigs aged >1 year combined. Distances were measured from the kill site to the nearest point on the western boundary of the study area. The lines are based on the predicted values for each pig based on the binomial regression models. The simplest linear model (i.e. based on just two age groups: <1 year or ⩾1 year) was: bTB prevalence=−0·31×distance+1·47×age group−0·16.
Pigs as temporal carriers
Of the 293 cases of M. bovis infection confirmed by culture, only eight had no visible lesions or equivocal pathology detected during necropsy, indicating that the vast majority of M. bovis cases presented with advanced (macroscopically detectable) lesions and that there were few cases of early stage infection in pigs aged >1 year. Across the total sample, bTB prevalence increased from 25·9% in pigs aged 0–1 years (95% CI 21·6–30·6, n=359) to 41·0% (95% CI 34·1–48·1, n=188) in pigs aged 1–2 years, and 52·3% (95% CI 44·1–61·2, n=127) in pigs aged 2–3 years, but was no higher in pigs aged >3 years (50·0%, 95% CI 40·5–59·5, n=102). Nine infected pigs were not aged. The pattern of an initially rapid rate of increase in infection prevalence, followed by a slowing-down of this rate of increase in old age, was not a consequence of control, as the same pattern occurred in pigs exposed only to uncontrolled possum populations (Fig. 3a).
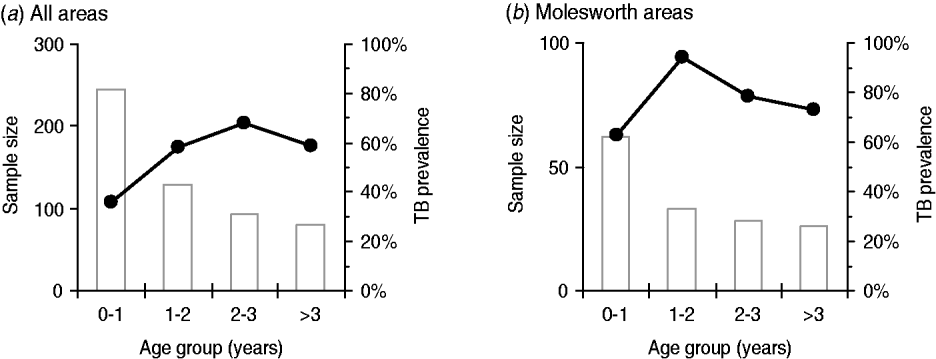
Fig. 3. Trends in age-specific prevalence in pigs from (a) all areas combined but excluding pigs collected after 2004/2005 possum control operations; and (b) from the three high prevalence areas on Molesworth Station (BH, DC, LM), again excluding post-possum control pigs.
The age-dependent infection pattern was most marked in pre-control pigs from the three heavily infected areas on MS (i.e. BH pre-2004, and DC and LM; Fig. 3b). There, almost all pigs up to age 2 years were infected: infection prevalence was 93·8% (95% CI 80·3–98·3, n=33) in pigs aged 1–2 years compared to just 73·1% (95% CI 53·9–86·3, n=26) in adults aged >3 years. This negative association between age and prevalence was significant (3×2 Fisher's exact test, P<0·001). Of the older pigs, 35% of the animals aged >3 years that presented with suspect TB lesions were classified initially as culture negative for M. bovis (n=43), compared to only 17% of those aged 1–3 years (n=103, Fisher's exact test, P=0·016). However, in some of the lesion-positive/culture-negative older pigs, the lesions were either heavily calcified or were fibrotic with few or no necrotic foci: hence, a retrospective lower dilution culture of the frozen reserve homogenate was conducted from these animals (a random subsample of 10 cases), and this detected low numbers of M. bovis bacilli present in 50% of these very advanced lesions (confirming that the initial figure of ~65% of lesion-positive >3-year-old pigs being M. bovis positive was a reasonably precise estimate).
DISCUSSION
There has been considerable debate as to the role of scavenging species (mustelids, pigs) in the acquisition and possible maintenance of M. bovis infection in New Zealand pastoral environments [Reference Coleman and Cook2, Reference Corner7, Reference Lugton8, Reference Cooke30]. One hypothesis has been that if bTB can be eliminated from the major reservoir species (possums) by population control then M. bovis will be eliminated among sympatric scavenging animals because infection cannot be maintained at the intra-species level. In the present report, we present results of bTB surveys in pig populations from a series of replicated studies in which possum population control was or was not applied, in order to support this hypothesis. However, it remains possible that other factors – including variance in genetic patterns of pathogen virulence and host susceptibility, and environmental factors such as host density and differences in social behaviour among pigs at different sites – could feasibly also have had a contributing role in the observed changes in bTB prevalence in pigs here.
We draw three main conclusions from our studies. First, there is strong evidence that bTB observed in feral pigs in New Zealand is acquired almost exclusively from possums (or other mammalian sources) rather than from other pigs, i.e. pigs are end hosts for the M. bovis transmission cycle in New Zealand. Once landscape-scale possum control was successfully applied, bTB prevalence in pigs declined to low levels within 2–3 years. As the infection prevalence in pigs often exceeded 30% in the absence of possum control, even modest levels of pig–pig transmission would have resulted in slower declines than those observed. The second main conclusion is that if bTB declined rapidly in pigs, it must have declined first (and therefore even more rapidly) in possums following population control; if it had not, then infected possum carcasses would have still been available to pigs for at least 2 years after control, and infected pigs still present for at least a few more years after that. We therefore infer that as possum density had fallen to a TCI <1% (or roughly <0·2 possums/ha) in response to full control in the WG and EH sites, the reproductive rate of the disease in that residual possum population declined to near zero, well below the level needed for bTB persistence. Our third conclusion is that pigs are ideal sentinels of bTB presence – they quickly develop a high prevalence of infection when infected possums are present, suggesting high sensitivity, but that ‘signal’ of bTB presence appears to dissipate within 2–3 years when the primary source of infection is removed.
The major management implication from this study is that rapid large-scale elimination of bTB from possums (and therefore, elimination of the transfer of infective M. bovis bacilli from possums to pigs) is not only feasible but is already being routinely achieved in New Zealand, since possum population control operations are routine practice for pest-management authorities. This finding is important because although it mirrors what is predicted by simulation models of bTB infection in possums, there is little other empirical evidence available to policy decision-makers to confirm that the strategy of large-scale intensive possum control can eliminate the disease locally in wildlife. In pigs, the increasing disease prevalence over the first 2–3 years of life observed in this study is in line with previous evidence that TB-induced mortality in feral pigs is low and that pigs can remain alive and infected for several years after first becoming infected [Reference Nugent, Whitford and Young31]. We therefore expect bTB to persist in pig populations for at least 4–5 years after possum control, i.e. until all of the cohorts born before possum control have died out. The observed declines occurred more quickly than this. The rapidity of these declines might indicate therefore that – in older pigs in particular – detectable bTB infection was disappearing rapidly in the absence of an external infection source. One possible explanation for this could be that the heavily encapsulated lesions observed frequently in pigs aged >3 years were beginning to resolve in these animals: the small decline in bTB prevalence into old age supports this supposition, as does the high occurrence of lesion-positive but culture-negative diagnoses in the oldest pigs. Resolution of infection is difficult to confirm without true longitudinal studies, but has been reported in possums [Reference Corner and Norton32], so is plausible for pigs given they are regarded as one of the host species that are best able to control the progression of clinical M. bovis disease [Reference Francis33]. There is therefore little evidence that already infected feral pigs provide a long-term reservoir of infection in New Zealand that would parallel the decade-long ‘temporal’ reservoir believed to result from lengthy survival times of other infected species, most notably wild deer [Reference Nugent6].
Internationally, the outcomes of this study are consistent with those obtained years earlier in the Northern Territory of Australia, where the prevalence of bTB in pigs declined from over 40% in some places [Reference Corner34] to very low levels (0·25%) when cattle and buffalo (which are undoubted maintenance hosts) were removed [Reference Radunz12]. As a result, Australia was declared officially free of bTB in 1997, and pigs are considered unequivocally as end hosts for the bTB transmission cycle in the Northern Territory [Reference McInerney, Small and Caley11, Reference Radunz12]. In sharp contrast, high levels (up to 100%) of TB-like lesions are present in wild boar on hunting estates throughout Spain and in at least one national park [Reference Parra16, Reference Gortázar35], and although infected red deer are usually also present there are some such estates where neither deer nor cattle are present, and yet TB-like lesions are still common in the wild boar. In other areas in Spain, free-ranging Iberian pigs and wild boar are infected with the same strains of bTB in areas where deer and cattle are rare or absent [Reference Parra16], further supporting the notion of intra-specific maintenance of bTB in wild suids in the Iberian Peninsula. In attempting to reconcile our results with the apparent ability of wild pigs overseas to maintain bTB infection independently, we focus on differences in pathology as a possible indicator that a different mode of transmission may be operating. In Australia and New Zealand, the predominance of head and alimentary tract lymphatic involvement in early stage infection suggests a predominantly oral route of infection as a result of scavenging infected carcasses [Reference Lugton8, Reference Gortázar35], although this in itself does not exclude the possibility of acquisition of primary infection via the airways, during scavenging, contributing to head-region lesion formation. In Spain, pulmonary lesion occurrence is reported far more frequently for TB in wild boar, perhaps indicating a higher incidence of respiratory tract-acquired infection in that case [Reference Hermoso de Mendoza36]. Vicente et al. [Reference Vicente18] suggest that pulmonary TB in Spanish pigs may be a consequence of the high densities and active management of wild boar on game estates in Spain, resulting in an increased frequency with which the animals interact in very close contact; additionally, Gortazar et al. [Reference Gortazar, Vicente and Gavier-Widén37] suggest that boar with infected salivary glands were likely to shed bacilli orally, thereby contaminating shared feeding and watering sites for animals in close contact. Increased density and supplemental feeding are strongly implicated as contributors to the emergence of self-sustaining bTB infection in wild white-tailed deer (Odocoileus virginianus) in Michigan, USA [Reference Miller38], and supplemental feeding of deer has been largely banned because of that. We therefore suggest that for wide-ranging sparsely distributed feral pigs in New Zealand, as in Northern Australia, the risk of intra-species disease transmission is seldom realized, presumably because close sharing of space is rare. Theoretically, maintaining pigs at much higher densities and clustering them together (e.g. at feeding stations) could be sufficient to convert them from spillover to maintenance hosts. However, a recent report by our group, studying disease transmission among wild-caught/pen-maintained bTB-infected pigs, has indicated that this is unlikely to occur, even at very high densities and with prolonged close contact [Reference Nugent, Yockney and Whitford39].
Data provided from this study indicate pigs as temporal carriers of bTB infection in New Zealand, and also provide evidence of spatial changes in infection related to localized possum relative abundance. In one region (EH study area) the possum population was progressively brought under control from east to west, over a 10-year period, by three successive poison operations; in doing so, bTB prevalence fell similarly among the sympatric pig population, until it eventually reached zero once the region had been fully controlled for possums (with the area to the west cleared last). Statistical analysis identified geographical location along that transect to be a significant predictor for bTB status in those pigs, along with age and time. These data, while not definitive, provide some evidence that as long as a population of M. bovis-infected possums remains at a moderate density, roaming and scavenging behaviour can similarly sustain bTB infection at a moderate prevalence in an adjacent pig population. From a practical disease surveillance point of view, this in turn implies that pigs will remain as good sentinels for spatially proximate disease in possums until that latter's population is brought under full control, confirming the utility of pigs as indicators of bTB in wildlife in New Zealand [Reference Nugent, Whitford and Young31].
In conclusion, this study has shown that M. bovis infection can be highly prevalent in feral pigs in New Zealand pastoral habitats, but that this prevalence is intimately linked to the disease status in concurrent possum populations: if the possums that maintain bTB are controlled, disease prevalence in pigs declines rapidly. In New Zealand, pigs serve as dead-end hosts for infection with the M. bovis bacillus, unlike the situation in other countries. As possum populations fluctuate in accordance with the level of control applied, adult pigs represent good sentinels for the prevalence of TB in the possum population, allowing for a temporal delay between the decline in possum numbers and monitoring of bTB in pigs.
ACKNOWLEDGEMENTS
We thank the main hunters who provided the bulk of the samples, particularly Bill Curnow, Phil Spencer, and Darren Foster. We also thank the land managers: Jim Ward of Molesworth Station; Colin and Tina Nimmo of Muzzle Station and the Department of Conservation. Andrea Byrom was a key co-researcher in related projects and with Mandy Barron provided useful comments on early drafts of the paper. Guy Forrester conducted the statistical analyses, and Christine Bezar helped with editing and formatting. The project was contracted by the Animal Health Board (Project nos. R80629, R10577 and R10688) and the New Zealand Foundation for Research, Science, and Technology (Contract C09X0009 and CO9X0803).
DECLARATION OF INTEREST
None.







