Campylobacter remains the most common cause of bacterial gastroenteritis in the UK. While the incidence of Salmonella infections has steadily declined since the late 1990s those caused by Campylobacter have remained high with almost 50 000 cases reported in England and Wales alone during 2008. Moreover, there has been an upward trend of reported cases with a marked increase of 17% observed during 2009 compared to 2008 [1]. The infection is unpleasant, although generally self-limiting; most patients experience acute enteritis for 7–10 days. Occasionally extra-intestinal infections or serious sequelae, including Guillain–Barré syndrome, and very rarely death, occur in about 1/1000 and 1/20 000 reported infections, respectively [2, Reference Humphrey, O'Brien and Madsen3]. The majority of clinical cases present as sporadic, with non-household outbreaks rarely identified.
In the UK, the risk factors for Campylobacter gastroenteritis, as identified by case-control studies and outbreak reports include consumption of inadequately cooked meat, untreated water, and unpasteurized milk. There is, however, strong evidence that suggests the handling of raw chicken and eating undercooked chicken is the most common cause of illness and the control of Campylobacter in poultry meat is a major public health strategy for the prevention of campylobacteriosis [2, Reference Humphrey, O'Brien and Madsen3]. In the UK, reducing Campylobacter in chicken has been a key target in the Food Standards Agency's strategy on foodborne disease since its inception in 2000, and will continue to be a focus in its new strategic plan for 2010–2015 [4]. The causes of the aforementioned recent increase of Campylobacter cases remain to be elucidated.
While outbreak exposures may differ to those of sporadic infections, they do provide essential source attribution information and support public-health and food-safety risk management and intervention strategies. We reviewed the frequency of general outbreaks (outbreaks affecting more than one household) of Campylobacter infection in England and Wales reported to the Health Protection Agency (HPA) from 1992 to 2009 and also examined transmission routes and implicated food vehicles.
Upon notification of general outbreaks of infectious intestinal disease in England and Wales, the HPA Centre for Infections obtains data from the outbreak investigations via a standard, structured questionnaire, and stores it in a dynamic database. We selected outbreaks of campylobacteriosis and analysed them with Microsoft Excel (Microsoft Corporation, USA) and Stata version 10 (StataCorp., USA). As defined by the European General Food Law Regulation [Regulation (EC) No. 178/2002], drinking water is now defined as a ‘food’. Relative proportions of outbreak settings and types were compared using the using the χ2 test. Changes in proportion with time were assessed using the χ2 test for trend.
Food was the predominant transmitter of Campylobacter (80%, 114/143), while person-to-person transmission (3%, 5/143) and animal contact (1%, 2/143) accounted for some of the remainder. The mode of transmission was unknown in 19 (13%) outbreaks. A total of 2676 people were affected in the 114 foodborne outbreaks, with 21 (1%) admitted to hospital but no deaths were reported. The mean size of the group affected was 23 (range 2–281, median 16, mode 4). Foodborne transmission was linked to food service establishments more frequently than other settings [64% (73/114) vs. 36% (41/114), P<0·0001], with 51% (37/73) of these linked to restaurants and 22% (16/73) to hotels. Notably, most of these outbreaks were associated with functions held at these venues [hotels, 88% (14/16); restaurants, 54% (20/37)].
Poultry meat was the most commonly implicated food vehicle in outbreaks (38%, 43/114), followed by drinking water (13%, 15/114) and drinking milk (7%, 8/114; 4 raw milk, 4 pasteurized milk (3 contaminated by bird pecking top of bottled milk, the other by pasteurization failure). The predominant poultry meat type and dish recorded in foodborne outbreaks was chicken (91%, 39/43) and liver pâté dishes [coarse (pâté) and smooth (parfait)] (58%, 25/43), respectively. Evidence implicating poultry liver pâté in outbreaks included microbiological evidence in three (12%), and analytical epidemiology and descriptive epidemiology evidence in six (24%) and 16 (64%), respectively. Food-handling faults reported in 68% (17/25) of these outbreaks showed that poultry liver pâté was prepared by deliberate undercooking (searing by flash frying) of livers or inadequate cooking of the blended livers in a bain-marie used in the preparation of this dish. Cross-contamination (24%, 6/25) and inappropriate storage conditions (4%, 1/25) also featured as contributory factors in outbreaks.
Campylobacter species colonize the gastrointestinal tract of a wide range of wild, domestic and livestock animals, including poultry, and the widespread high levels (>60%) of contaminated raw poultry meat and animal livers with Campylobacter is well documented [Reference Kramer5, Reference Little6]. Studies have also shown that pathogens such as Campylobacter may be present throughout the liver tissue, i.e. both on the outside and the inside of chicken liver, and inadequate cooking can result in viable pathogens remaining in the end product [Reference Whyte, Hudson and Graham7, Reference Baumgartner8]. This reinforces the need to cook poultry livers and other varieties of animal offal until a safe internal temperature is reached (and they are no longer bloody at the core). As these poultry liver pâté dishes are a fatty food, they may also favour the passage of Campylobacter through the gastric acid barrier. The survival of even small numbers of campylobacter in liver pâté dishes could be sufficient to cause illness [2]. Pâté dishes made from meat livers have also been associated with outbreaks of Salmonella and Listeria infections [Reference Threlfall, Hall and Rowe9, Reference de Valk10].
Significantly, an upward trend in the proportion of outbreaks linked to poultry liver pâté consumption occurred from 2007 [2007–2009, 74% (14/19); 1992–2006, 12% (11/95)] (P<0·00001). A greater proportion of Campylobacter outbreaks were also associated with consumption of poultry liver pâté dishes during December (Fig. 1) compared to all other months collectively (50% vs. 19%, P=0·013); while other Campylobacter outbreaks displayed the seasonal peak in May and June. This typical seasonal peak in Campylobacter infections is well recognized and is thought to be associated with the rise in indigenous temperatures [Reference Tam11], whereas that observed with the liver pâté outbreaks is suggestive of increased consumption outside the home around the Christmas period. The increase in the proportion of outbreaks associated with poultry liver pâté dishes has been concurrent with a decline in those linked to milk over the 18-year period (P=0·001). This may be the result of the decline in either raw drinking milk sales at the farm gate (permitted in England and Wales) and/or doorstep sales of bottled milk (reducing the likelihood of contamination via bird pecking) given the dominance of the retail food sector [12, 13]. In addition, no Campylobacter outbreaks linked to drinking-water supplies have been reported to the HPA since 2002 (15 in total between 1992 and 2002; 10 associated with private water supplies and five with public water supplies [system failures included breakdown of disinfection system (3) and influx of agricultural pollution (2)]. Since the identification of Campylobacter outbreaks is rare [5·1% (143/2759) reported between 1992–2009], the observed increase in those linked to poultry liver pâté consumption is significant and appears to be associated with the current deliberate undercooking of poultry liver pâté dishes. Caterers and consumers must recognize that this culinary trend of superficially cooking and serving poultry liver ‘pink’ means that any Campylobacter present will not have been destroyed [Reference Whyte, Hudson and Graham7, Reference Baumgartner8]. There is no specific market research information on consumption of poultry liver pâté dishes to determine whether there has been a trend in increased consumption of this dish. However, expenditure on eating out in the UK has declined since 2005; this downward trend covers most food categories including meat dishes [14]. Recent Campylobacter outbreaks linked to chicken liver pâté consumption has also been reported in Scotland and New Zealand, and similarly highlighted the fault of insufficient cooking during preparation of the dish [Reference Whyte, Hudson and Graham7, Reference Forbes15, Reference O'Leary16].
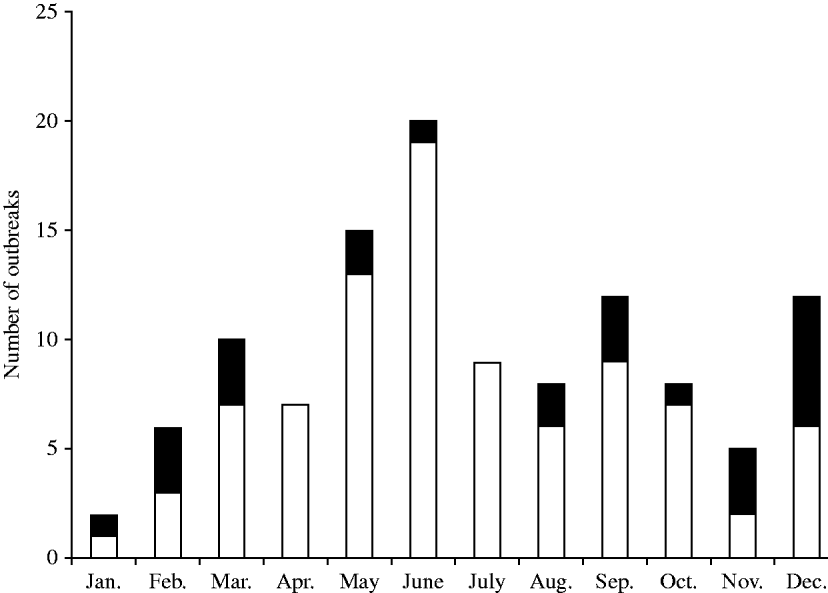
Fig. 1. Seasonality of foodborne outbreaks of campylobacteriosis (□) and specifically those associated with consumption of poultry liver pâté (▪), England and Wales, 1992–2009.
More attention needs to be paid to food-handling practices in food service establishments to lower the risk of Campylobacter infection. The present study identifies important risk factors that are modifiable through changes in behaviour, particularly when they relate to cooking and hygiene practices that favour the survival of the pathogen or cross-contamination during the preparation of food in the kitchen. Since 2006 food business operators have been required to produce and follow written food safety procedures and should regularly monitor and review them to ensure their procedures are being followed [Regulation (EC) No. 852/2004 on the hygiene of foodstuffs]. These procedures ensure that food is purchased, stored, prepared and served safely. A key part of that is to ensure that meat such as animal offal, including poultry liver, is thoroughly cooked before consumption (to a core temperature of 70°C for at least 2 min or equivalent using a meat thermometer to check the core temperature).
The number of Campylobacter outbreak cases continues to constitute a small proportion of notifications reported to the HPA. The nature of the organism and its epidemiology make outbreaks difficult to detect and investigate and this has been compounded by the lack of follow-up of Campylobacter infections and infrequent referral of isolates to reference facilities in England and Wales [2, Reference Humphrey, O'Brien and Madsen3]. Systematic molecular typing would help to distinguish one strain of Campylobacter from one another and/or trace sources to outbreaks but further refinement of methods and agreement on the best suitable technique are needed.
ACKNOWLEDGEMENTS
The authors thank all reporting investigators in England and Wales including Health Protection Units (England), The National Public Health Service for Wales (NPHS) (Wales), Microbiologists, Local Authorities and Environmental Health Officers for their continual support in reporting to the electronic foodborne and non-foodborne gastrointestinal outbreaks surveillance system. The authors also thank I. A. Gillespie, S. LeBaigue and C. Penman for their previous maintenance of the database at HPA.
DECLARATION OF INTEREST
None.



