INTRODUCTION
Non-typhoid Salmonella are an important cause of enteric diseases and are associated with severe morbidity and excess mortality worldwide [Reference Helms1].
Salmonellosis is a foodborne disease, commonly manifested by acute enterocolitis, with sudden onset abdominal pain, diarrhoea, nausea, sometimes vomiting and fever [Reference Heymann2]. Symptoms usually appear within 1–3 days following exposure and persist for 3–5 days. Serious bloodstream infections can occur, particularly in the very young or in the elderly, who are also at the highest risk for morbidity and mortality, depending on the non-typhoid serotypes [Reference Weinberger3, Reference Jones4]. The extent of morbidity and mortality associated with non-typhoidal salmonellosis revealed the need for enhanced surveillance to follow sporadic (about 60–80% of all cases [Reference Heymann2]) and outbreak source cases. Surveillance networks such as FoodNet and EnterNet have been established in the USA and Europe aiming at early detection of enteric disease outbreaks, detection of changes in the epidemiology of the different agents causing these outbreaks and identifyng new genes encoding resistance to antibiotics.
The FoodNet surveillance run by the CDC's Emerging Infections Program reported a decrease in the incidence rate of Salmonella laboratory-confirmed infections when comparing 1996–1998 and 2007 data [5]. A decrease was also demonstrated in Europe by Denny et al. [Reference Denny6] and in England and Wales [Reference Adak7].
The incidence of salmonellosis in Israel increased from 31·6/100 000 in 1954 to 109·1/100 000 in 1996, decreased thereafter to 16·0/100 000 in 2005 and increased to 20·3/100 000 in 2009 [8].
Increasing antimicrobial resistance among Salmonella has been noted in several countries in the last decade [Reference Cailhol9, Reference Martin10]. The incidence of multidrug-resistant S. Typhimurium and mostly multidrug-resistant S. Typhimurium DT104 rose markedly worldwide, including in Israel, during the 1990s and has thus become an increasing public health problem around the world [Reference Ford11–Reference Metzer13].
A sentinel laboratory-based surveillance network was established in Israel in 1997 by the Israel Center for Disease Control (ICDC), which integrates information on Salmonella isolates from community and hospital laboratories and from the Salmonella National Reference Laboratory.
In this paper we present data generated by this network on agent and host-dependent characteristics of Salmonella enteric infections and antibiotic resistance patterns occurring in Israel in the last decade.
MATERIALS AND METHODS
The sentinel laboratory-based surveillance network for bacterial enteric diseases (salmonellosis, shigellosis, campylobacteriosis) was established in Israel in 1997. Five community laboratories and three hospital-based laboratories representing the different geographical regions of Israel participate in this network: Haemek Medical Center (HMC) and Haifa HMO (HH) in northern Israel, Sheba Medical Center (SMC), Maccabi Dan District Community Laboratory (MD) and Clalit HMO Petah-Tikva (CPT) in central Israel, Hadassah (Ein Karem and Mount Scopus) (EK and MS) medical centres and Meuhedet Community Laboratory (MCL) in Jerusalem District and Soroka Medical Center (SMC), Beer-Sheba in southern Israel. Clalit HMO Petah-Tikva Community Laboratory was added to the network in 2004. The population served by the five community laboratories (HMC, HH, MD, MCL, SMC) is well defined and covers 30·7% of the total Israeli population. The data presented in this paper refer to these five community laboratories for which the size and age distribution of the population they served was known and made possible the calculation of incidence rates. Demographic data on individuals with a positive stool sample for Salmonella and isolate characterization to the serogroup level were obtained from the sentinel laboratories and transferred to the ICDC, where they were integrated with the data on the further characterization of the isolates at the Salmonella National Reference Laboratory of the Ministry of Health.
The Salmonella National Reference Laboratory performed serotyping, phage-typing and antibiotic resistance testing. In the mid-2009, the performance of phage-typing was discontinued. This paper includes phage-type data until 2008.
Salmonella of the same serogroup and serotype isolated from the same individual within a month were counted only once. This paper refers to non-typhoidal Salmonella isolates.
Susceptibility of Salmonella isolates of the serotypes S. Enteritidis, S. Typhimurium, S. Virchow and S. Hadar to ampicillin (AMP), sulfamethoxazole–trimethoprim (SXT), ceftriaxone (CRO), chloramphenicol (C), ciprofloxacin (CIP) and tetracycline (TET) was determined at the Salmonella National Reference Laboratory of the Ministry of Health, by the Kirby–Bauer disk diffusion method [Reference Bauer14]. The tests were performed on Muller–Hinton medium using Oxoid antimicrobial susceptibility disks (Oxoid, UK). Multidrug resistance was defined as resistance to three or more antimicrobial agents. These tests were performed for the years 2002–2007. The data on antibiotic resistance are presented for the total eight sentinel laboratories.
Data analysis
Data analysis was performed using SAS software (release 9.1.3; SAS Institute Inc., USA). Information on the population size served by each sentinel laboratory was available by age group and sex. Incidence rates were calculated by year, month, age group and for the five most prevalent serotypes by dividing the number of individuals positive for Salmonella by the population covered by that laboratory.
We investigated time trends in the incidence rates in Israel from 1999 to 2009, using linear regression models.
RESULTS
Between 1 January 1999 and 31 December 2009, data on 8758 isolates of Salmonella from stool specimens were reported to the ICDC by the five community laboratories according to the following breakdown: HH 2417 isolates (27·6%), SMC 1791 (20·4%), MCL 2046 (23·4%), MD 1783 (20·4%) and HMC 721 (8·2%).
Age and gender distribution
Data on patient's age was achievable for 8649 Salmonella isolates. A total of 5558 (64·3%) isolates were from children in the 0–4 years age group. The proportions of isolates from the 5–14, 15–24, 25–34, 35–44, 45–54, 55–64 and ⩾65 years age groups were 11·7% (1011 isolates), 5·4% (468), 4·7% (403), 3·1% (271), 3·6% (311), 2·7% (236) and 4·5% (391), respectively. Of children aged <5 years, 1683 (30·3%) were isolated in the first year of life, 2123 (38·2%) in the second year of life, 975 (17·5%) in the third and 777 (14·0%) in the fourth and fifth years of life. Of the 8620 isolates for whom data on patient's gender were available, 53·2% (4587 isolates) were from male patients.
Salmonella serogroups, serotypes and phage types
The five most commonly identified serogroups were: Salmonella C1 (26·2%), Salmonella D (26·1%), Salmonella B (17·8%), Salmonella C2 (12·5%) and Salmonella E (2·0%). Of the total isolates, 11·8% were identified just by biochemical features and reported as Salmonella spp. without specification of serogroup.
The ten most commonly identified serotypes over the period 1999–2009 were: S. Enteritidis (26·5%), S. Virchow (12·7%), S. Typhimurium (8·8%), S. Infantis (8·4%), S. Hadar (7·3%), S. Bredeney (3·3%), S. Montevideo (3·0%), S. Java (2·2%), S. 9,12:l,v:- (2·1%) and S. Newport (1·9%). S. Enteritidis was the most commonly isolated serotype in all age groups.
Routine phage-typing was performed for S. Enteritidis and S. Typhimurium using the Israeli phage-typing scheme. The three most prevalent phage types among S. Enteritidis were B3 (47·4%), F3 (21·5%) and C8 (18·5%). The three most prevalent phage types among S. Typhimurium were 2(4+) (44·6%), Group 2 (22·3%) and R9 (9·0%).
Time trends
There was a significant decrease in the incidence rate of Salmonella isolates from 70·5/100 000 in 1999 to 21·6/100 000 in 2005 (P value<0·01) followed by an increase to 30·3/100 000 until 2009 (P value=0·03 for trend) (Fig. 1). This time trend was evident in all five community laboratories (data not shown). The decrease in the incidence rate of Salmonella isolates from 1999 to 2005 and the increase from 2005 and onwards were consistent in all age groups and in both males and females (data not shown). The highest incidence rates throughout the years were observed in the 0–4 years age group, which also experienced the most significant decrease from 437·7/100 000 in 1999 to 121·0/100 000 in 2005 followed by and increase to 176·4/100 000 in 2009. Other age groups showed the same trends with about tenfold lower incidence rates.
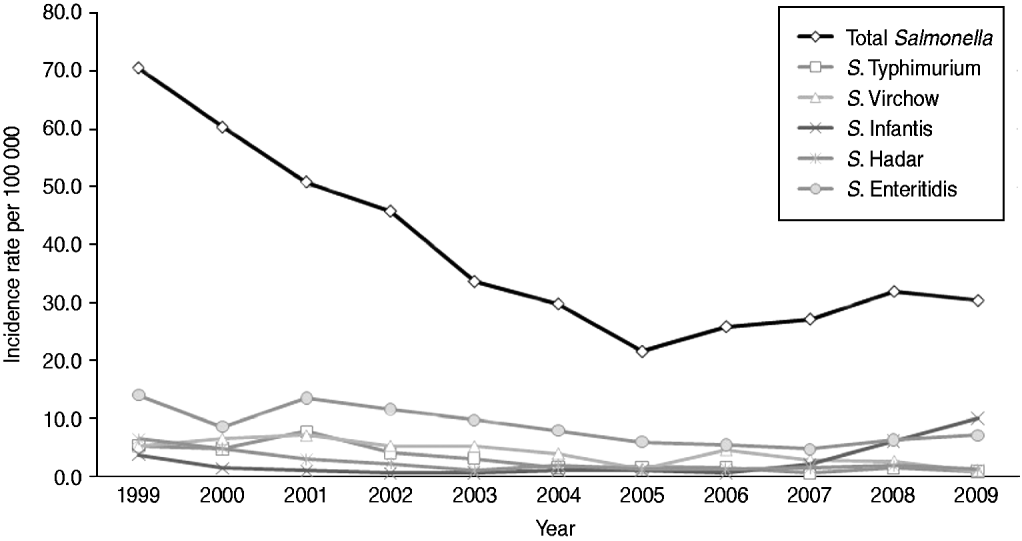
Fig. 1. Incidence rate (per 100 000) of total Salmonella isolates and five most commonly identified Salmonella serotypes in five sentinel community laboratories, 1999–2009.
There was a clear pattern of seasonality in the number of Salmonella isolates over the years with an increase in April–May, a peak in August and a decrease thereafter. A shifting in the most prevalent serotype was observed in 2009. The incidence of S. Enteritidis, the most prevalent serotype during 1999–2008, decreased from 14·0/100 000 in 1999 to 4·7/100 000 in 2007 and increased to 7·1/100 000 in 2009. The incidence of S. Infantis increased markedly from 0·6/100 000 in 2006 to 10·0/100 000 in 2009, becoming the most prevalent Salmonella serotype in Israel in 2009 (Fig. 1).
Antibiotic resistance
Between 2002 and 2007, 1490 (59·8%) of 2491 isolates were tested for antibiotic resistance (617 S. Enteritidis, 435 S. Virchow, 219 S. Typhimurium, 219 S. Hadar). The proportions of antibiotic resistance to these antibiotics are presented in Table 1. S. Typhimurium was highly resistant to tetracycline, ampicillin and chloramphenicol. S. Virchow was resistant to tetracycline, sulfamethoxazole–trimethoprim and chloramphenicol. S. Hadar was resistant to tetracycline. Over 95% of the S. Enteritidis isolates were susceptible to all six antibacterial drugs. Of 1490 isolates tested for antibiotic resistance, 15·4% were multidrug resistant (resistant to three or more antimicrobial agents). Multidrug resistance was found in 37·9% of S. Typhimurium strains, 32·6% of S. Virchow strains, 0·5% of S. Enteritidis and in 0·5% of S. Hadar strains.
Table 1. Proportions of antibiotic resistance patterns of Salmonella serotypes, 2002–2007
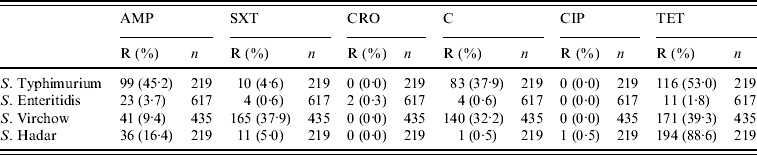
R, Resistant; AMP, ampicillin; SXT, sulfamethoxazole–trimethoprim; CRO, ceftriaxone; C, chloramphenicol; CIP, ciprofloxacin; TET, tetracycline.
Common antibiotic resistance patterns are presented in Table 2. The most frequent pattern (36·1%) belonged to S. Typhimurium isolates which were resistant to ampicillin, chloramphenicol and tetracycline (R-Type ACT). Another common resistance pattern was that of S. Virchow isolates which simultaneously showed resistance to sulfamethoxazole–trimethoprim, chloramphenicol and tetracycline (25·8%).
Table 2. Antibiotic resistance patterns of Salmonella serotypes, 2002–2007
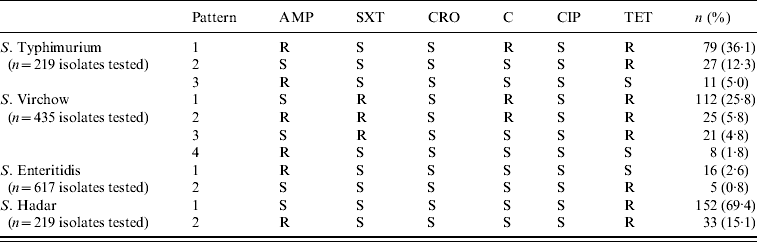
AMP, Ampicillin; SXT, sulfamethoxazole–trimethoprim; CRO, ceftriaxone; C, chloramphenicol; CIP, ciprofloxacin; TET, tetracycline.
DISCUSSION
In this article we report on 11-year data generated by a sentinel, laboratory-based surveillance network of salmonellosis established in Israel in 1997. These data show that the incidence of salmonellosis in Israel decreased markedly from 70·5/100 000 in 1999 to 21·6/100 000 in 2005, and increased to 30·3/100 000 in 2009. The parallel incidence rates reported by the Department of Epidemiology of the Israel Ministry of Health based on the national passive surveillance system which was established at the beginning of the 1950s, were 63·0/100 000 in 1999, 16·0/100 000 in 2005 and 20·3/100 000 in 2009 [8]. Although both systems of surveillance identified a similar trend in the incidence of salmonellosis in Israel, the figures yielded by the national passive surveillance system were lower and probably reflect under-reporting. Moreover, by integrating data on the isolation of Salmonella at the sentinel laboratories with information related to patients (gender, age, address, etc.) and characterization of isolates at the National Reference Centers of the Ministry of Health, the sentinel laboratory-based surveillance could show that the decrease in the incidence of non-typhoidal salmonellosis encompassed both genders and especially children aged 0–4 years and could display the changes in the relative importance of the leading Salmonella serotypes throughout 11 years of surveillance.
A decrease in the incidence of non-typhoidal salmonellosis in the last decade was also seen in other countries. According to FoodNet, the incidence rate of Salmonella laboratory-confirmed infections decreased by 8%, comparing data from 1996–1998 and 2007 [5]. Data from Denny et al. showed a similar trend [Reference Denny6]. In England and Wales, the estimated cases of non-typhoidal Salmonella fell to less than half between 1992 and 2000 [Reference Adak7]. The incidence of Salmonella infections in Israel is still higher than that reported from other developed countries. In the USA, the reported incidence rate was 14·9/100 000 in 2007 [5], in comparison with 27·2/100 000 in the present study, for the same year. We cannot rule out the possibility that this difference could be at least in part due to differences in referral of specimens, testing for pathogens or other reasons.
Salmonella was consistently most common in children aged <5 years. Similar age distributions have been reported in the USA [5, Reference Olsen15–17] and Australia [Reference Owen18]. The reason for the high incidence rate in this age group has been described previously [Reference Mountzouris19] and includes the high susceptibility of the intestinal microflora since the immune system is still developing and because this age group is more likely than others to expose infected items to their mouths.
We have shown a marked pattern of seasonality of salmonellosis, with incidence rates rising in the warm months and decreasing in the cold months, as expected. This seasonal pattern was also demonstrated in the USA [Reference Olsen15, 16] and Canada [Reference Ford11].
S. Enteritidis was the most common serotype detected of all serotypes in the 11 years of surveillance. Other prevalent serotypes were S. Virchow, S. Typhimurium, S. Infantis, S. Hadar, S. Bredeney, S. Montevideo, S. Java, S. 9,12:l,v:- and S. Newport, by rank. According to the FoodNet Emerging Infections Program, in 2007, the most common serotypes in the USA were: S. Enteritidis, S. Typhimurium, S. Newport, S. 1,4,[5],12:i:-, S. Javiana, S. Heidelberg and S. Montevideo [5]. The WHO Global Salm-Surv country databank, where 31 countries submitted data on 125 745 human Salmonella isolates worldwide, reported that during 2002, S. Enteritidis was also the most common serotype among humans, accounting for 65% of all isolates [Reference Galanis20]. After S. Enteritidis, the nine most common serotypes in humans were S. Typhimurium, S. Newport, S. Heidelberg, S. Infantis, S. Hadar, S. Virchow, S. Javiana, S. Saintpaul and S. Montevideo, by rank [Reference Galanis20].
S. Enteritidis is related to foodborne outbreaks and sporadic cases of human gastrointestinal diseases worldwide [Reference Fernandes21] are mostly associated with undercooked eggs [Reference Delarocque-Astagneau22–25]. In Germany, the decline in the incidence rate of Salmonella between 2001 and 2008 was related to a decrease in the incidence rate of S. Enteritidis [Reference Frank26].
The Public Health Laboratory Information System (PHLIS), a national Salmonella surveillance system, which collects data on reported laboratory-confirmed Salmonella isolates in the USA, reported that S. Typhimurium and S. Enteritidis both declined substantially (28% and 34%, respectively) between 1995 and 2005 [17]. The most marked decrease in our study was observed for S. Typhimurium, consistently throughout the surveillance period, with an incidence rate of 5·3/100 000 in 1999 to 0·8/100 000 in 2009. The FoodNet surveillance system in the USA also reported a decrease in the incidence of S. Typhimurium [5]. In Europe, a decline in S. Enteritidis was observed between 1998 and 2005 [Reference Fisher27] and in S. Typhimurium between 1998 and 2003 [Reference Fisher28].
The decrease in the incidence of S. Typhimurium is of particular importance due to the high rate of antibiotic resistance acquisition of this serotype.
Between 1999 and 2006, S. Infantis was ranked fifth among Salmonella serotypes in Israel. Since December 2007, the incidence of this serotype increased markedly and in 2009, S. Infantis became the most prevalent Salmonella serotype in Israel. Worldwide, S. Infantis was ranked fifth among Salmonella serotypes in 2002 [Reference Galanis20] and in the USA, S. Infantis was ranked ninth between 1987 and 1997 [Reference Olsen15].
The significant decrease in salmonellosis caused by S. Enteritidis and S. Typhimurium and the emergence of S. Infantis in Israel as the most prevalent serotype parallel similar changes in the incidence and distribution of Salmonella serotypes occurring at the level of the main natural reservoir of Salmonella, i.e. poultry. Actions taken to reduce S. Enteritidis and S. Typhimurium infections in poultry as tight surveillance of all breeding flocks and hatcheries, culling or treatment of all flocks infected with S. Enteritidis or S. Typhimurium, improvement of biosecurity and infrastructure, and systematic immunization against S. Typhimurium and S. Enteritidis started in 1994 and 1996, led most probably to the marked decrease in the incidence of these serotypes in poultry. In 1996, S. Enteritidis or S. Typhimurium were isolated from 33·6% of the breeding flocks and in 2009 only 0·4% were positive to one of these two pathogens (E. Berman, personal communication). Since 2008, S. Infantis has been the most frequent serotype among breeding flocks, accounting for 27·9% of all poultry between June 2008 and May 2009 (E. Berman, personal communication). The temporal association between the increase in incidence of S. Infantis in breeding flocks and in humans and the similar pulsed-field electrophoresis profiles of S. Infantis human and poultry isolates [Reference Gal-Mor29] reveal the important role of the animal reservoir and the foodborne transmission of Salmonella in Israel.
The incidence rate of S. Virchow infections was ranked second over the 11 years of surveillance in Israel. The high prevalence of S. Virchow among non-typhoid Salmonella serotypes is unique to Israel [Reference Weinberger30]. In the USA and Europe, S. Virchow accounts for <1% and <5% of total non-typhoid Salmonella isolates, respectively [Reference Weinberger30] and in 2002, S. Virchow was ranked only seventh among human Salmonella isolates worldwide [Reference Galanis20]. Physicians should pay attention to isolation of S. Virchow since it tends to invade the bloodstream and to cause invasive infection, especially in infants aged <2 years [Reference Weinberger3]. We will follow this pattern to discover whether S. Enteritidis will regain its position of leading importance among the Salmonella serotypes isolated in Israel.
The data we provide on the distribution of the S. Enteritidis and S. Typhimurium phage types are based on the Israeli phage-typing scheme, which prevents international comparisons, except for the incidence of S. Typhimurium phage type 2(4+) (according to the local scheme) which was documented as being identical to DT104 (definitive phage-type 104) [Reference Metzer13]. The proportion of this S. Typhimurium phage type declined from 56·0% in 1999 to 35·1% in 2008. The reduction of DT104 phage type is of public health importance due to the resistance of this specific phage type to multiple antibacterial drugs.
We found that S. Typimurium and S. Virchow were more resistant to antibiotics compared to S. Enteritidis and S. Hadar. S. Typimurium and S. Virchow were also highly multidrug resistant. High rates of multidrug-resistant S. Virchow were also reported in Denmark for the period 2003–2005 [Reference Bagger-Skjøt31].
In spite of being the most prevalent serotype, S. Enteritidis did not acquire antibiotic resistance over the years, as documented by a comparison of the findings of a study conducted in southern Israel between 1994 and 1996, when over 95% of S. Enteritidis isolates exhibited susceptibility to various antibiotic drugs [Reference Metzer13]. Reports from the USA also indicate a low resistance rate of S. Enteritidis [32]. During 2002–2007, the period for which antibiotic resistance patterns are presented in this paper, S. Infantis was not tested routinely for antibiotic resistance. However, data on S. Infantis resistance patterns in Israel including 71 randomly selected isolates of S. Infantis which were identified in Israel during 2007–2009 (21 human sources, 28 poultry sources, 22 food sources) and were tested for antibiotic resistance showed that the dominant clone of S. Infantis was multidrug resistant (96%), with a combined resistance pattern to nalidixic acid, nitrofurantoin and tetracycline, with or without additional resistance to trimethoprim–sulfamethoxazole [Reference Gal-Mor29].
It should be borne in mind that the laboratory-based surveillance system underestimates the real burden of salmonellosis. Only a fraction of individuals with gastroenteritis consult a physician, and only a small part of them have a stool culture performed. A population survey performed by FoodNet reported a ratio of 1:39 between laboratory-confirmed cases and Salmonella infections in the community [Reference Voetsch33].
In conclusion, despite the significant decrease in incidence rates over the last 11 years, Salmonella infections are still common in Israel with specific emerging serotypes such as S. Infantis which have become more prevalent than S. Enteritidis and S. Typhimurium. There is still a clear need for improvement of hygiene and sanitary standards in breeding flocks and hatcheries. It is expected that these actions, coupled with enhanced education of food handlers and consumers with regard to prevention of foodborne disease transmission will further decrease the incidence of salmonellosis in Israel.
ACKNOWLEDGEMENTS
We thank Nadia Pekurovski for her assistance in the data collection of the sentinel laboratory-based surveillance network and Anneke Ifrah for her contribution in reviewing this study summary.
DECLARATION OF INTEREST
None.





