Antimicrobial cycling, the scheduled use of specific antibacterials or antibacterial classes rather than others, reportedly prevented morbidity and mortality due to nosocomial Gram-positive and Gram-negative antimicrobial-resistant infections in a surgical intensive care unit (SICU) at the University of Virginia [Reference Raymond1]. Due to insufficient data documenting efficacy, antimicrobial cycling, is not currently recommended by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America as a method to reduce antimicrobial resistance [Reference Dellit2]. While some investigators have reported that cycling was associated with lower rates of antimicrobial resistance, as mentioned above, others have reported that it was not associated with lower rates of antimicrobial resistance [Reference Warren3].
Multiple studies examining Stenotrophomonas maltophilia colonization or infection have found an association between therapy with broad-spectrum antibiotics and S. maltophilia colonization or infection [Reference Villarino4, Reference Carmeli and Samore5]. Imipenem was one of the broad-spectrum antibiotics shown to have such an association. Carmeli et al. conducted a historical cohort study to determine whether imipenem therapy posed a bigger risk for nosocomial S. maltophilia infections than did use of ceftazidime therapy [Reference Carmeli and Samore5]. There was a greater risk of S. maltophilia acquisition when both agents were used than when imipenem or ceftazidime was used alone, but monotherapy with imipenem appeared to pose a similar risk to that of monotherapy with ceftazidime. Carbapenem use was an independent risk factor for a high number of S. maltophilia isolates per 1000 patient-days in 39 intensive care units that were part of the Surveillance of Antimicrobial Use and Antimicrobial Resistance in German Intensive Care Units surveillance system [Reference Meyer6].
A cluster of S. maltophilia isolations was noted in the same University of Virginia SICU as reported by Raymond et al. [Reference Raymond1] during an imipenem cycling protocol implemented for control of presumed Gram-negative nosocomial infections. This prompted a retrospective study of the rate of S. maltophilia colonization and/or infection during periods of carbapenem vs. non-carbapenem cycling with those during non-cycling.
The University of Virginia Hospital (UVH) is a 600-bed tertiary care referral centre. Between 1999 and 2002, various antibiotic cycling protocols were used in the Medical Intensive Care Unit (MICU) and the SICU. During cycling periods, carbapenems were included as first choices for empirical therapy during 3-month rotations of antimicrobials covering Gram-negative bacteria. Other antibiotics included in the cycling regimens were ciprofloxacin, cefepime, and piperacillin/tazobactam. During some cycling periods there were two different antibiotic classes being cycled, depending upon the suspected Gram-negative infection. For example, if pneumonia was suspected, piperacillin/tazobactam was selected, if infection of unknown origin was suspected, then cefepime was the chosen cycled antibiotic. Over the study period, patients with S. maltophilia were not cared for in isolation. After 1996 patients that were not in isolation received care using Standard Precautions according to Centers for Disease Control and Prevention (CDC)'s 1996 Isolation Guideline.
Computer records of the Clinical Microbiology Laboratory were used to identify all positive cultures for S. maltophilia from January 1992 to December 2002. Cultures obtained from patients on the paediatric, obstetric, and psychiatric units as well as a clinical research ward were excluded from the analysis because patients from these units generally had very low rates of S. maltophilia colonization at UVH. For each positive culture of S. maltophilia, the following were recorded into a Microsoft Access database: date, body site of the specimen, and the patient care unit in which the patient resided when the specimen was obtained. Any patient having at least one culture positive for S. maltophilia was counted as being colonized with S. maltophilia. If an individual patient had multiple cultures positive for S. maltophilia, only the first isolate was included in the analysis.
Incidence density rates of new S. maltophilia colonizations were calculated for each unit. To assess differences in incidence rates between antibiotic cycling periods and non-cycling periods, several comparisons were made. For both the SICU and MICU, the primary unit locations of antibiotic cycling, incidence density rates were compared between periods of no antibiotic cycling and periods where the non-carbapenem classes of antibiotics were cycled and periods when the carbapenem class of antibiotics was the sole rotating antibiotic class or part of the rotation. Another comparison was conducted focusing on three other surgical units possibly affected by antibiotic cycling in the SICU (due to regular transfer of patients between these units and sharing of the same staff who may have prescribed the antibiotics being cycled to their non-SICU patients as well). A similar comparison was made with the MICU and three other medical wards. A final analysis was conducted of annual incidence rates of new S. maltophilia colonizations per 100 patient admissions for the entire hospital population from 1992 to 2002.
Rates of S. maltophilia nosocomial infection per 100 patient admissions over the study period were obtained from available Hospital Epidemiology computer records covering the years 1993–2002 in order to assess for a secular trend across the hospital. The wards surveyed for nosocomial infections were the same as those for which microbiology records were assessed as described above except that three wards with very low infection rates had routine surveillance for nosocomial infections cancelled due to a cutback in hospital support of Hospital Epidemiology in the final months of the final analysis year 2002 (i.e. a cardiology ward and hospice unit in August 2002, and an additional cardiology ward in October 2002). Definitions of nosocomial infections were those published by the CDC [Reference Garner7]. These definitions were updated to reflect changes in CDC system definitions throughout the study period, such as for surgical site infections in 1992 and for pneumonia in January 2002. Infection control practitioners performed surveillance hospital-wide for nosocomial infections using the Kardex method throughout the study period as previously described [Reference Wenzel8].
Incidence rates of S. maltophilia colonization between antibiotic cycling periods and non-cycling periods for the SICU, MICU, other surgical wards, and other medical wards were compared using methods appropriate for comparing incidence densities, the large-sample two-sample inference test and the exact test [Reference Rosner9]. A χ2 test for linear trend was used to assess yearly incidence rates of colonization and of nosocomial infection per 100 patient admissions for three different time periods: (1) for the hospital population over the whole study period; (2) for the pre-cycling period (1992–1998); and (3) for the cycling period (1999–2002). The statistical software, EpiInfo version 6 (CDC, USA) was used; P<0·05 was considered to be statistically significant. This study was approved by the Human Investigation Committee of The University of Virginia.
A total of 1326 cultures were positive for S. maltophilia from the entire hospital population; 697 isolates were excluded from analysis because they represented subsequent isolates from patients with a previous isolate of S. maltophilia. Of these 629 remaining isolates, the majority of the positive cultures were from respiratory specimens (75%). Of the unique 629 cultures hospital-wide positive for S. maltophilia, there were a total of 129 cultures positive from the SICU and 112 from the MICU. The results of the comparisons between antibiotic cycling periods (SICU: 1999–2002; MICU: August 2001–2002) and non-cycling periods (SICU: 1992–1998; MICU: 1992–July 2001) are shown in Table 1. No statistically significant difference was found between rates for antibiotic cycling periods and non-cycling periods in the SICU, other surgical wards, MICU or other medical wards.
Table 1. Relative risk of S. maltophilia colonization during different time periods
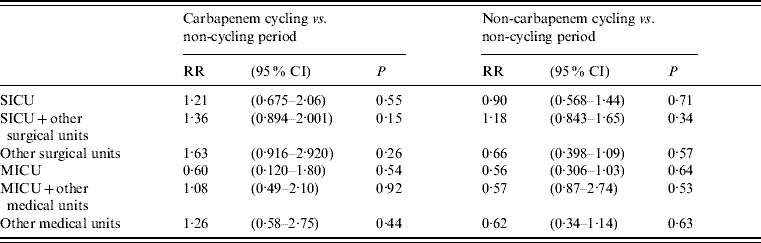
RR, Relative risk; CI, confidence interval; SICU, surgical intensive care unit; MICU, medical intensive care unit.
For the results of the three linear trend tests: (1) there was a trend towards a decrease in S. maltophilia colonization hospital-wide over the 11-year study period (P=0·07013, Fig. 1); (2) during the 7-year pre-cycling period, there was a significant decline in colonization (P=0·01572); and (3) there was a trend towards an increase from 1999 to 2002 when cycling was being used in the SICU and/or MICU (P=0·11791).
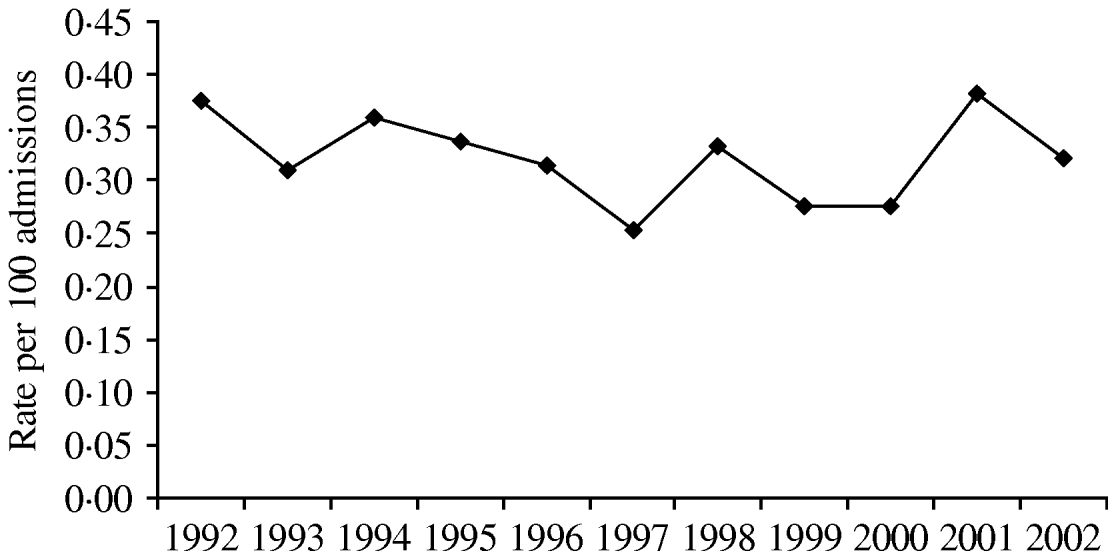
Fig. 1. Hospital-wide annual rates of S. maltophilia (number of isolates/100 patient admissions) from January 1992 to December 2002.
According to Hospital Epidemiology computer records, there were 130 nosocomial infections due to S. maltophilia from 1993 to 2002; of these, 90 were polymicrobial. The relative frequency distributions of the 130 infections were: respiratory tract (54%), blood (19%), urinary tract (15%), surgical site (7%), eyes, ears, nose (2%), abscess (2%), and bone (1%). There was evidence of a significant increase in the rate of nosocomial infection over time from 1993 to 2002 (P=0·01728), from 0·45 infections per 1000 patient-days in 1993 to 0·57 in 2002.
Our study did not address the issue of whether a cycling programme results in overall control of antimicrobial resistance, but focused instead on a limited question: whether cycling antimicrobial agents having a broad spectrum of activity against Gram-negative bacteria was associated with any change in the frequency of colonization or infection by S. maltophilia, an organism naturally resistant to many antimicrobial agents. While we found no significant change in the isolation rate of S. maltophilia from clinical cultures during periods of antibiotic cycling, the incidence of S. maltophilia colonization was 21–36% higher in the SICU and SICU step-down units during cycling (Table 1). It should be noted that cycling was conducted in the SICU for twice as long as in the MICU, thus possibly affecting SICU rates of colonization more than MICU rates. There was no significant difference found between periods of non-cycling and cycling with any of the broad-spectrum regimens used for Gram-negative infections, including piperacillin/tazobactam, cefepime, and ciprofloxacin, nor was there any significant difference between periods of non-cycling and cycling with the use of carbapenems. Quarterly antibiotic cycling rotations for the treatment of Gram-negative infections as used at UVH may not result in increased rates of S. maltophilia, although statistical power may have been insufficient to detect a 21–36% increase as significant.
Although the rate of colonization by S. maltophilia as detected by positive microbiology cultures did not vary significantly between periods of cycling and non-cycling, there was a significant increase in the rate of nosocomial infections due to S. maltophilia during the study period. The hospital-wide increase in infections seemed to occur over the 11 years studied rather than just the last 4 years in which cycling regimens were being used in the SICU and/or MICU, so there may be multiple reasons for the observed increase in infection beyond any possible effects of cycling. One possibility is that there was an increasingly more ill patient population that was perhaps more immunosuppressed and/or had a higher relative frequency of use of invasive devices or duration of use of invasive devices that posed a risk for S. maltophilia once colonized with this organism. Such confounding variables could explain an increase in S. maltophilia infection in the absence of an increase in colonization rates. It also should be noted that colonization rates were based upon positive routine clinical cultures that probably detect only a minority of all colonized patients. If clinical cultures do not provide a reliable index of all colonized patients, then it is possible that colonization rates actually rose and that this might have contributed to the observed increase in nosocomial infections.
An important limitation of this study was the inability to assess the quantity of antibiotics actually used during periods of cycling and non-cycling. Cycling may or may not increase the use of the cycled agent. Adherence to cycling regimens can be problematic [Reference Dellit2]. Additional studies of cycling are needed that account for actual usage of particular antimicrobial agents. Another limitation in the interpretation of findings was the use of two different antibiotic rotation regimens during two of the SICU cycling protocols for the treatment of pneumonia and peritonitis/infection of unknown origin. The use of two different antibiotic regimens per rotation may have decreased the selection pressure favouring S. maltophilia. A possible explanation for no change in colonization rates with the cycling protocols could be due to ‘streamlining’ of antibiotic therapy. In all of the protocols, antibiotics could be streamlined to narrower spectrum agents following positive cultures for Gram-negative organisms if the antibiotic chosen was in the same class, or in the case of carbapenems, if the antibiotic chosen was of narrower spectrum but was being cycled in other quarters. If protocol antibiotics were frequently switched to narrower spectrum agents, then antibiotic-resistance selection pressure could have been less than if the cycling antibiotic had been continued.
As subsequent isolates were excluded in this analysis, it was not possible to assess the possibility of multiple colonizations with S. maltophilia for individual patients. It remains possible that a higher frequency of multiple infections per patient could have occurred during periods of antibiotic cycling. This is an area that warrants further study, as over half of the total number of S. maltophilia isolates (697) was excluded from the analysis. Further, the relatively small number of S. maltophilia isolates during any one month or quarter in any unit lead to relatively low statistical power for comparisons between individual quarters. Pooling the data for all cycling quarters and for those with particular drugs provided additional power, but the negative results for individual units could still be due to limited power. The hospital-wide rates give the advantage of increased statistical power because of larger numbers, but the disadvantage of including many units that were not using cycling at all and thereby diluting any potential effect of the cycling on S. maltophilia rates.
Finally, even if significant differences had been found between S. maltophilia colonization or infection rates between non-cycling and cycling periods, it would not have been possible to directly attribute outbreaks of S. maltophilia to antibiotic pressure in the hospital. Nosocomial outbreaks of S. maltophilia colonization or infection have occurred without detectable change in antibiotic selection pressure due to a contaminated environmental reservoir (e.g. dialyser effluents, an ice-making machine, etc.) [Reference Denton and Kerr10]. Thus, it would be difficult to attribute a clinically significant increase in the rates of S. maltophilia colonization or infection to antibiotic cycling protocols without thorough investigation of any detected epidemic to exclude other potential causes. S. maltophilia colonization did not change significantly after implementation of antibiotic cycling protocols at UVH, but the potential to detect a significant difference may have been limited by low statistical power. More research is needed to determine whether antibiotic cycling protocols affect antimicrobial resistance rates, including S. maltophilia infection rates.
ACKNOWLEDGEMENTS
The authors thank Sandy-Getchell White and Eve Gianetta for assistance in obtaining computer records used for analysis in this study.
DECLARATION OF INTEREST
None.




