INTRODUCTION
A novel reassortant avian influenza A(H7N9) virus was detected in China in February 2013 [Reference Gao1]. It led to 552 human infections and about 200 deaths by 23 February 2015 in mainland China [2]. The reported case numbers are still increasing [Reference Liu3]. Evidence supports the origination hypothesis of triple reassortment between H9N2 virus and H7 and N9 viruses [Reference Lam4–Reference Liu7].
The widespread incidence of H9N2 virus in Chinese poultry stock provides an ideal reassortment environment for H7N9 virus [Reference Liu8, Reference Wu9]. Previous studies verified the contribution of H9 virus in the process of H7N9 virus evolution [Reference Yu10, Reference Liu11]. However, to our knowledge, no study has assessed the role of H9 virus in relation to the risk of H7N9 virus contamination in the environment. In this study, we assessed the risk of H7N9 virus contamination in environments associated with H9 virus based on a surveillance programme from March 2013 to April 2015 in Zhejiang province, China.
METHODS
Specimen and data collection
A surveillance programme on H7N9 virus in poultry related to the environment has been in effect since the first H7N9 case emerged in March 2013 in Zhejiang province. The province consists of 11 prefectures, which are further divided into 90 counties and finally into 1329 towns. In each prefecture, one-third of the counties were surveyed each month, thus all counties could be covered quarterly. As shown in Figure 1, 568 out of the 1329 towns in Zhejiang province were surveyed from March 2013 to April 2015.

Fig. 1. Distribution of towns surveyed for H7N9 virus in Zhejiang province, China, March 2013–April 2015.
The surveillance premises included live poultry markets, poultry-rearing farms, concentrated areas of backyard poultry farms, slaughtering and processing plants, habitats for migratory birds, and other poultry-related sites (such as living quarters of human H7N9 cases, restaurants, supermarkets, and markets for cold fresh poultry). In each prefecture, at least 30 specimens were collected monthly for each type of sample, which included poultry faeces, poultry cage swabs, drinking water for poultry, sewage from cleaning poultry, and swabs of tables used for slaughtering or holding poultry.
The number of H7N9 cases was collected from the China Information System for Disease Control and Prevention [Reference Wang12].
Laboratory testing
Viral RNA was extracted from specimens with the RNeasy mini kit (Qiagen, USA), and real-time RT–PCR was then performed with H7N9-specific primers and probes according to the manufacturer's protocol. The cycle threshold was defined as ⩽37·0. Real-time RT–PCR was performed at 11 local influenza network laboratories of Zhejiang province to detect H7, N9 and H9 viruses, and validated by the Zhejiang provincial influenza network laboratory [13, 14].
Statistical analyses
Student's t test was used to compare the mean positive rate of H7N9 virus between towns or premises with positive H9 virus detected and those with negative results. Fisher's exact test was used to compare the positive rates of H7N9 virus in different environmental specimens. Spearman's correlations were calculated for the positive rate of H7 virus and the number of H7N9 cases aggregated monthly. Multivariate logistic regression was used to calculate the adjusted odds ratios (aOR), with 95% confidence intervals (CI) as a measure of association between the H9 and H7N9 viruses. Stratified analyses and interaction terms were used to examine the effect of modification of H9 virus in areas, premises, prefectures, surveillance years and sample types. All analyses were performed with PASW software v. 18.0.0 (http://pasw.en.malavida.com/).
RESULTS
Of 568 towns under surveillance in Zhejiang province from March 2013 to April 2015, 207 tested positive for H9 virus and 175 for H7N9 virus. The mean positive rate of H7N9 virus in towns positive for H9 virus (10·87%) was higher than for those without H9 virus (3·80%) (t = −5·418, P < 0·001). Of 1723 premises distributed in the 568 towns surveyed, 369 tested positive for H9 virus and 248 for H7N9 virus. The mean positive rate of H7N9 virus was 12·12% in premises positive for H9 virus and 2·84% for those that were negative (t = −7·852, P < 0·001).
Of 16 275 environmental specimens collected, 1400 (8·6%) and 959 (5·9%) tested positive for H7 and H7N9 viruses, respectively. The number of H7N9 cases counted monthly was significantly correlated with the positive rate of H7 virus (r = 0·411, P = 0·033) (Fig. 2). The positive rates of H7N9 virus were much higher in specimens acquired in 2015, from live poultry markets, in Hangzhou and Huzhou prefectures, in sewage used for cleaning poultry and on tables used for slaughtering or holding poultry (detailed information of H7N9 virus for all specimens summarized by year, prefecture and sample types provided in Supplementary Table S1). Moreover, the positive rate of H7N9 virus in environmental specimens with positive H9 virus was over four times higher than for those that were negative (Table 1).

Fig. 2. The correlation of the positive rate of H7 virus and the number of H7N9 cases in Zhejiang province, China, March 2013–April 2015.
Table 1. Positive rates of the H7N9 virus in different environmental specimens, Zhejiang Province, China, March 2013–April 2015
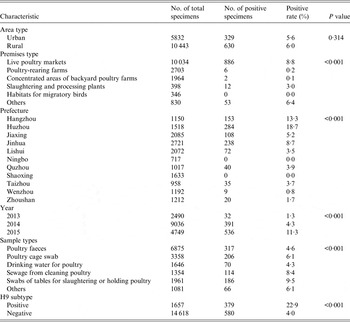
Associations between characteristics of environmental specimens and H7N9 virus contamination are shown in Table 2. When we analysed premises and prefectures, we combined some categories with similar H7N9-positive rates because of their small number. Multiple logistic analyses showed that type of premises, prefecture, surveillance year and sample type were confounding factors in the association between H9 and H7N9 viruses in environmental specimens. There was a significant association between H9 and H7N9 viruses (aOR 4·49, 95% CI 3·79–5·31) after adjustment for these confounding factors.
Table 2. Odds ratios for associations between environmental specimens and H7N9 virus contamination, Zhejiang province, China, March 2013–April 2015

cOR, Crude odds ratio; aOR, adjusted odds ratio; CI, confidence interval.
As shown in Table 3, a significant multiplicative interaction (P < 0·001) was found between area and H9 virus, with nearly a fivefold increased risk of H7N9 contamination in environments of rural areas positive for H9 virus compared to urban areas with no H9 virus detected (aOR 4·93, 95% CI 3·90–6·23). The expected aOR for the combination of rural area and H9 virus was 3·40 in the additive model and 3·25 in the multiplicative model.
Table 3. Odds ratios for H7N9 virus according to H9 virus and characteristics of the environment, Zhejiang province, China, March 2013–April 2015

aOR, Adjusted odds ratio; CI, confidence interval.
There was also significant multiplicative interaction (P < 0·001) between the premises and H9 virus. The risk of H7N9 virus contamination associated with the combination of H9 virus and live poultry markets (aOR 46·80, 95% CI 31·83–68·81), was higher than that expected in the additive model (aOR 34·10) and was one-sixth of that expected in the multiplicative model (aOR 280·26).
In prefectures, we also identified a significant multiplicative interaction (P < 0·001). The OR of H7N9 virus contamination in environments with H9 virus in Huzhou prefecture was nearly seven times that in environments without H9 virus in Hangzhou prefecture (aOR 6·86, 95% CI 4·61–10·21). The estimate for the combination of Huzhou prefecture and H9 virus was more than twice that expected from adding (aOR 2·94) and multiplying (aOR 2·94) the independent associations.
Risk of H7N9 virus contamination in environments with H9 virus in 2014 and 2015 was significantly higher than in those without H9 virus in 2013; the aORs were 7·73 (95% CI 5·03–11·87) and 40·67 (95% CI 27·11–61·02), respectively. The expected aORs for environments positive for H9 virus in 2014 and 2015 were 1·58 and 4·77 in the additive models, respectively, and were both zero in the multiplicative models.
Regardless of sample type, the aORs of H7N9 virus for positive H9 specimens were much higher than for negative H9 specimens. When compared to poultry faeces without H9 virus, the aOR of H7N9 virus contamination increased from 5·58 to 26·15 across all specimen types with H9 virus.
DISCUSSION
To our knowledge, this study was the first to assess the association of H9 virus with the risk of H7N9 virus contamination in the environment. We found that environments positive for H9 virus had significantly higher risk of H7N9 virus contamination than those that were not. Risk of H7N9 virus contamination associated with positive H9 virus was much greater in 2015 and in rural areas, live poultry markets, Huzhou prefecture and sewage used for cleaning poultry.
H9 virus plays an important role in the reassortment of H7N9 virus in China [Reference Liu7, Reference Cui15]. The co-existence of H9 and H7N9 viruses in an environment will generate appropriate conditions for reassortment, leading to rapid evolution of H7N9 virus, which in turn enables it to change its genetic architecture rapidly, increasing viability in the environment and transmissibility in poultry or humans. The six internal genes of H7N9 virus, which originate from the H9N2 virus that circulates in chickens in eastern China [Reference Liu7, Reference Kageyama16, Reference Lam17], might affect the infectivity of H7N9 virus [Reference Feng18]. A recent study showed that a genotype (G57) of H9N2 virus had changed antigenicity and improved adaptability to chickens, which facilitated the genesis of the H7N9 virus [Reference Pu5]. Our study found that the presence of H9 virus was associated with an almost fivefold increased risk of H7N9 virus contamination in the environment. This implies that we might be able to predict epidemics caused by H7N9 virus by monitoring the prevalence and variation of H9 virus [Reference Pu5]. Moreover, the risk of H7N9 virus prevalence could also be decreased by reducing the amount of H9 virus in the environment.
Our study showed that the combination of a rural area and H9 virus was associated with an almost fivefold increased risk of H7N9 virus contamination in the environment. We also found that the H7N9 virus risk associated with rural areas was null in environments with no H9 virus detected. This indicates that the risk of H7N9 virus contamination in rural areas is the same as in urban areas if the H9 virus is absent. This interaction effect implies that H9 virus control is helpful for H7N9 virus control in rural areas.
Evidence based on the exposure histories of cases [Reference Li19, Reference Zhang20] and similarity of gene sequences [Reference Ke21] supports the conclusion that live poultry markets are the main sources of H7N9 virus infections in humans [Reference Li22]. In China, the special environment of live poultry markets, including the polyculture of a number of hosts in a high-density setting, provides ideal conditions for reassortment and transmission of the H7N9 virus [Reference Liu23]. Interestingly, our study shows that the independent risk of H7N9 virus contamination associated with H9 virus is much higher than that associated with live poultry markets, and that the combination of H9 virus and live poultry market led to a 47-fold increased risk of H7N9 virus contamination in the environment. If this finding can be replicated, it would mean more effort is needed to monitor the prevalence of H9 virus and to explore the effects of measures to reduce the levels of H9 virus in the environment.
Zhejiang province was one of the worst-hit areas of the H7N9 epidemic in China. Huzhou prefecture in Zhejiang province, located in the geographical centre of the Yangtze River Delta region, is considered one of the regions of H7N9 virus origin [Reference Ling24]. Our study indicated that H9 virus in the environment in Huzhou prefecture led to a high risk of H7N9 virus. The co-circulation of avian influenza virus subtypes H7, H9, N2 and N9 in poultry in Huzhou prefecture provides an ideal dynamic environment for the ongoing generation of H7N9 virus [Reference Han25, Reference Han26]. The risk of H7N9 virus contamination in the environment in Huzhou prefecture could be similar to that in Hangzhou prefecture, neglecting the influence of H9 virus.
Evolutionary temporal dynamics suggested that there were at least two rounds of reassortment events during the generation of H7N9 virus. The N9 gene was first reinserted into the H9N2 genome. The H7 gene was then reassorted into the genome of the reassortant intermediate H9N9 virus [Reference Han25]. Benefiting from the short cycle of reassortment and the wide range of co-existence for H9 and H7 viruses, the amount of H7N9 virus in the environment increased rapidly. This study found a rapid increase in risk for H7N9 virus from 2013 to 2015 in environments with H9 virus. Moreover, this grave situation could be exacerbated if this co-circulation of H9 and H7 viruses continues without control. Unfortunately, due to absence of detection of the N2 gene in the surveillance programme, we were unable to learn the distribution of the parental H9N2 virus and the evolutionary intermediate of the H9N9 virus, which restricted a detailed assessment of the evolutionary consequence of co-circulation of H7N9 and H9N2 viruses.
In summary, this study provided a quantitative association between H9 virus and increased risk of H7N9 virus contamination in the environment. The prevalence of H9 virus in the environment will significantly increase the risk of H7N9 virus. Moreover, it might continue to be a source of further genetic diversity for H7N9 viruses [Reference Liu27]. In light of these findings, we recommend that the gene variability of the H7N9 and H9 viruses should be constantly monitored. Moreover, effective measures are required to control environments favouring reassortment of H7N9 virus, especially those with co-circulation of H7N9 and H9 viruses.
SUPPLEMENTARY MATERIAL
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/S0950268816002168.
ACKNOWLEDGEMENTS
We thank 90 county CDCs for their field investigation and sample collection, and 11 prefecture CDCs for their assistance with laboratory testing in Zhejiang province.
This study was supported by the Zhejiang Provincial Major Science and Technology Programme (2014C03039) and the Key Platform Programme of Zhejiang Medical Technology (2015ZDA009). None of the funders had any role in the study design or the collection, analysis, and interpretation of data, or in the writing of the article and the decision to submit it for publication. The researchers confirm their independence from funders and sponsors.
DECLARATION OF INTEREST
None.








