INTRODUCTION
European bat lyssaviruses (EBLVs), family Rhabdoviridae, genus Lyssavirus, are most probably transmitted to humans by biting. In The Netherlands, the reservoirs of EBLV-1 are found to be the Serotine bat, Eptesicus serotinus, and for EBLV-2 the pond bat, Myotis dasycneme, but the Serotine bat is by far the most important reservoir [Reference Van der Poel1]. The prevalence in The Netherlands of EBLV-1 fluctuates around 20% of the submitted Serotine bats [Reference Van der Poel1]. In the past decades, five single fatal cases of bat rabies in humans caused by EBLV have been reported from respectively Ukraine (1977, 2002), Russia (1985), Finland (1985), and Scotland (2002) [Reference Fooks2–Reference Botvinkin4].
Among the native mammal species in The Netherlands involved in biting humans, bats are the leading cause for administering post-exposure rabies vaccinations. Between 1987 and 1992, 174 people in The Netherlands were vaccinated after suspected contact with native bats [Reference Nieuwenhuijs, Haagsma and Lina5] and between 1997 and 2003, 148 people were vaccinated [Reference Van Riemsdijk6]. In Denmark, 10 individuals received prophylactic treatment for possible exposure to bat rabies in 2003 [Reference Christensen and Olsen7].
Rabid bats can transmit the virus to domestic as well as to wild non-chiropteran mammals, but records of bat rabies in non-chiropteran mammal species in Europe are rare. Thus far, transmission to five sheep in Denmark [Reference Tjørnehøj8] and to a Stone marten, Martes foina, in Germany [Reference Müller9] has been reported. From a survey of domestic cats in Denmark it was estimated that 1–3% of cats were EBLV seropositive [Reference Tjørnehøj, Rønsholt and Fooks10]. Domestic cats do, indeed, prey on numerous small animals, including bats [Reference Woods, McDonald and Harris11]. Although probably rare, indirect transmission of EBLV to humans via domestic and wild mammals might be possible.
The risk for humans contracting bat rabies after any contact with a rabid bat depends partly on the frequency and nature of the exposure to infected bats. For the purpose of assessing the risk of bat rabies to humans, we report the frequency and the nature of bat incidents in humans, as well as in cats and dogs. In addition, a mathematical model is developed to evaluate the dose-dependent risk of human bat rabies infection. Although we identified that essential information is still lacking, this study was undertaken to show a concept of how to assess the risk of bat rabies in humans after exposure by rabid bats.
MATERIALS AND METHODS
Bat specimens
Passive surveillance of lyssaviruses in bats has been undertaken in The Netherlands since 1984. Grounded bats that were unable to fly and bats reported to have been in contact with humans and/or pets were submitted to the Central Institute for Animal Diseases Control (CIDC–Lelystad) for detection of lyssavirus antigen. This surveillance was carried out nationwide from 1987 to 1994 but thereafter testing for EBLV was largely restricted to suspected rabid bats, and bats involved in contact with humans and/or pets. Brain tissues were collected from all bats submitted between 1984 and 2005. Over 100 specimens from different bat species were normally tested for EBLV annually. Thirty bats that were diagnosed positive for EBLV by polymerase chain reaction (PCR) and fluorescent antibody test (FAT) in a 3-year period were used to determine the presence of the virus in salivary glands and neck skin.
Detection of lyssavirus
Bats were tested for EBLV by standard FAT as described by Dean et al. [Reference Dean, Abelseth, Atanasiu, Meslin, Kaplan and Koprowski12] with minor modifications, using polyclonal fluorescein isothiocyanate-labelled rabbit anti-rabies nucleocapsid IgG (Diagnostics Pasteur, Marnes-la-Coquette, France). Mice were inoculated intracerebrally with clarified brain tissue suspensions originating from either an experimentally infected cat (genotype 1) or an infected Serotine bat from the field (genotype 5). Mice were killed after clinical symptoms appeared. Brain tissue smears of these mice were used as positive controls in the FAT. Duplicate smears were carefully and completely checked for fluorescence. For amplification of EBLV-specific RNA, tissue samples (3 mm3) were placed in 0·5 ml RNA extraction buffer. The RNA extraction was performed using TRIzol. TRIzol (1 ml) was added to the tissue sample and RNA extraction was performed according the manufacturer's protocol (TRIzol, Invitrogen Life Technologies, Merelbeke, Belgium). Reverse transcription (RT) and PCR amplification were performed as described by Heaton et al. [Reference Heaton13] followed by Southern blot hybridizations of RT–PCR products as described by Van der Poel et al. [Reference Van der Poel14].
Geographical analysis
The species, gender, and age of each bat were identified by external body features and, together with the determined finding date and location (5×5 km grid), inserted in a database. The database consisting of a total of 1271 records of the Serotine bat was used for geographical analysis. For Serotine bats collected in 2000–2005, the nature of contacts with humans and animals was also determined and added to the database for contact analysis. The locations of collected bats were visualized using ArcGIS 9 (ESRI, Redlands, CA, USA). The population density was calculated from 1219 Serotine bat records for the period 1984–2004 [Reference Van der Poel1] with the program Spatial Analyst (ESRI) using a kernel density and a search radius equal to 30 km.
Risk of EBLV
We describe the number of humans bitten by any Serotine bat in a given year as a stochastic process by using a Poisson distribution with the average number of biting incidents equal to λ. Since we assume that the majority of bite incidents do not lead to rabid disease, we denote by the symbol p the probability of developing human bat rabies upon being bitten by a Serotine bat. In this situation, the number of human bat rabies cases when a random number of biting incidents occurs (determined by the Poisson distribution with the rate λ) is Poisson distributed with the new rate λp, i.e. it is a Poisson mixture of a binomial distribution [Reference Johnson, Kemp and Kotz15].
The probability of developing rabies upon being bitten by a Serotine bat depends on the person's susceptibility to the virus and the number of infectious EBLV viral particles transmitted into the body through the biting wound. We assume that a single viral particle can, although not necessarily, cause human rabies with a small probability r, which can be deduced from experimental infection with EBLV-1a in mice [Reference Vos16] using a single-hit dose–response model [Reference Haas17, Reference Teunis and Havelaar18]. The single-hit dose–response model is often applied for ingested infectious material, i.e. foodborne pathogens, but its mathematical derivation does not depend on the specific mode of entrance into the host body and therefore the theory can be applied to infection caused by the bite of an infected Serotine bat. The number of virus particles in the saliva is expected to vary between individual Serotine bats; some Serotine bats might not excrete the virus at all, others do only in low numbers and some in higher numbers. We denote by the symbol f(i) the likelihood that the number of the viruses excreted in a unit volume (e.g. 1 μl) of the bat's saliva is equal to i. When i virus particles in a unit volume are transmitted through the open wound, the probability of at least one virus particle inflicting the disease in the victim is equal to 1 – (1 – r)i. Because i can be any non-negative integer we sum this quantity weighted by the likelihood f(i) to obtain the risk of human rabies upon being bitten by a Serotine bat.
Because no experimental data is available to estimate f(i), we consider the best and the worst cases. The risk of bat rabies is the highest if all infected bats excrete a large number of the viruses in excess of human LD50 [=r −1 ln (2)]. Denoting by k a number in excess of human LD50, the risk of bat rabies approaches the probability of being bitten.
High estimate:
Assuming all infected bats excrete only one virus particle, we would obtain a low estimate of risk:
Seasonal change in the prevalence
We describe the number of rabid bats by the binomial distribution with the prevalence that can either be a constant or be specific to summer and winter months. The benefit of assuming season specific prevalence is tested by the likelihood ratio test. The deviance (twice the difference of log-likelihood values) is incorporated into the χ2 distribution with 1 degree of freedom to calculate the P value.
RESULTS
Contact incidents
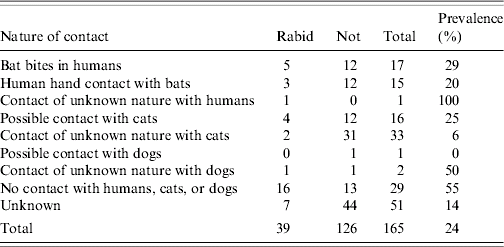
Between 2000–2005 encounter of humans and pets with Serotine bats varied in frequency and contact type (Table). In this period 17 humans were bitten by bats, 15 touched bats and one person had a contact of unknown nature. Five of the 17 bats that bit people were EBLV positive (29%). EBLV was detected in 3/15 bats involved in only hand contact (20%) as well as in a single bat that had a contact of unknown nature. Overall prevalence of EBLV in Serotine bats coming into close contact with humans is 27% (9/33) consisting of biting (5/17), hand contact (3/15), and a contact of unknown nature (1/1) (Table). Cats had the most frequent contacts with bats (49 times including suspected contacts; Table). However, only six (12%) of these bats were shown to be positive for EBLV. Contacts of dogs with bats were reported three times only. Serotine bats that did not have contact with humans or pets were more likely to be EBLV positive (16/29=55%). Bat incidents with humans and with cats were reported nationwide but incidents with EBLV-positive bats were clustered in the northern half of the country (Fig.).

Fig. Bat contact incidents 2000–2005. (a) Incidents of human individuals bitten by Serotine bats or having touched Serotine bats by hand are indicated by solid circles (●, EBLV negative) or by open triangles (△, EBLV positive). The density of EBLV-positive Serotine bats in the environment is indicated by the grey shading; darker shading represents higher density. (b) Incidents of cats contacting or suspected of contacting Serotine bats are indicated by solid circles (●, EBLV negative) or by open triangles (△, EBLV positive).
Table. Incidents and contact types with Serotine bats in 2000–2005
Seasonal prevalence
Bat contact incidents were reported in all seasons but more often in summer months when bats are exhibiting more ‘out-roost’ activity than during the hibernation period. The total monthly reports between 2000 and 2005 amounted to 165 incidents with highest number of reports in July and August. In this period a total of 39 bats were found to be rabies positive with again the highest numbers in July and August (8/30 and 9/38 positives, respectively). Regardless of contact type, the chance of contacting EBLV-positive Serotine bats between April and October (38/134=28%) was significantly higher than during the hibernation period between November and March (1/31=3%, P=0·998).
Presence of EBLV in different body parts
EBLV was present in the neck skin of 20/30 brain tissue-positive bats (67%) and in medulla oblongata in 27/28 brain tissue-positive bats (96%). Most importantly for the risk of bat rabies by biting, EBLV was present in the salivary glands of 22/30 brain tissue-positive bats (73%). In order not to underestimate the risk of bat rabies, we assume that all brain tissue-positive Serotine bats do excrete one or more EBLV particle in the saliva.
Risk of human bat rabies
A total of 17 bats that bit humans were identified in 6 years in the entire country (Table). The numbers of bites reported in each year are 1, 5, 3, 1, 3, and 4, ordered chronologically from 2000 to 2005. The mean number of reported bites per year (2·8) is approximately equal to the variance (2·6), justifying our assumption that the number of reported bites per year is Poisson distributed. According to national statistics [19], a total of 16 305 526 individuals lived in The Netherlands in 2005. Thus the average exposure rate based upon the reported biting incidents is 2·8 bites per year (λ) per population of 16 million. We estimate the probability that an infectious EBLV particle inflicts human rabies (r) based on experimental EBLV infections using mice [Reference Vos16]. In five mice intramuscularly injected with 102·5 f.f.u. (foci-forming units) of EBLV-1a, two mice died. With 104·5 f.f.u., 5/5 mice died. Using a single-hit dose–response model [Reference Haas17, Reference Teunis and Havelaar18] the maximum-likelihood estimate of the probability r is equal to 1·6×10−3 per injected virus. To estimate f(i) the likelihood that the number of EBLV excreted in the saliva of individual Serotine bats is equal to i, we note that 12/17 Serotine bats that bit humans were EBLV negative. Thus f(0)=12/17=0·7. Unfortunately it is not known what exactly the number of EBLV particles was in the five EBLV-positive Serotine bats that did bite humans. In addition, there is no information available, even in the literature, regarding the concentration of EBLV in the saliva of Serotine bats.
To make a maximum estimate for the risk, we assume that all five bats were excreting a large number of EBLV viruses in excess of human LD50. Then by equation (1) the probability of acquiring human bat rabies upon being bitten by a Serotine bat is p=1−f(0)=1−0·7=0·3. Thus, the estimated risk of human bat rabies infection is λp=2·8×0·3≈0·8 per year on average. The number of human bat rabies cases in a given year varies according to a Poisson distribution with the rate 0·8 per year. In a 14-year period, one would expect 6 years of no human bat rabies cases, 5 years of one case, 2 years of two cases, and 1 year of three or four cases. More than five human cases are unlikely to occur. Thus it is estimated that as a mean about one case per year would occur when there is no post-exposure prophylaxis. This is the worst-case scenario.
If the five EBLV-positive bats transmitted only one EBLV particle, we would obtain a low estimate of risk. Using equation (2) we obtain
That means human bat rabies might occur at the rate equal to λp≈1·4×10−3 per year, equivalent to once in 700 years on average in The Netherlands, a country of 16 million residents.
DISCUSSION
The estimated risk of EBLV from a bat biting based upon the reported contacts with bats and the EBLV prevalence among the bats involved in the contact incidents still ranges widely between 1 per year and 1 per 700 years in a population of 16 million residents. In taking a systematic approach to the public health implication of EBLV, this study identified the most important information gap: to reduce uncertainty in the estimated risk of rabies infection of Serotine bat origin, a quantification of EBLV in the saliva of these bats is needed. Active surveillance in Serotine bats [Reference Serra-Cobo20] or experimental EBLV infection in Serotine bats could fill the information gap. EBLV is generally believed to be excreted in the saliva in a low concentration, but only limited data on this subject are available [Reference Harris21, Reference Vos22]. If this is correct, the risk of bat rabies following a bite by a rabid bat would still be present but negligibly small [Reference Morris23]. However, if low amounts of EBLV are shed by saliva, it might be difficult to explain how EBLV can circulate in Serotine bat populations, unless one considers a vertical transmission or other routes that do not involve biting, e.g. infection of the mucous membrane via aerosols or by licking. In an experimental infection vampire bats excreted ~103 rabies virus particles (genotype 1) per ml of saliva on only one occasion and remained apparently healthy until the experiment ended on the 710th day [Reference Aguilar-Setien24]. Relevance of this study to our study is however limited because the rabies virus genotype and the bat species used to perform the experiment were different and because extremely high viral doses were used in that experimental infection. Nonetheless it cannot be excluded that rabid Serotine bats may excrete a high concentration of EBLV. A total of 103 EBLV is sufficient to cause neurological disorder and death in about a half of the mice by the intramuscular route [Reference Vos16]. It might be clear that for the purpose of risk assessment, more quantitative information is needed on the number of EBLV viral particles transmitted by saliva or other body fluids.
The Serotine bat is relatively common in The Netherlands and can be found throughout the whole country. It lives mainly alongside humans in cavity walls of houses and other buildings. The population density is the highest in the northern part of the country, especially in the north-western part. In the southern part it is present but in low numbers [Reference Kapteyn, Limpens, Mostert and Bongers25]. This suggests that the population density in the southern part is below the critical density at which EBLV is able to persist.
Records of contacts between cats and EBLV-positive bats are relatively low. The reason why most cats have no contact with EBLV-positive bats is yet to be resolved, but from some observations we know that rabid bats sometimes screech against approaching subjects or emitted sounds, and this may deter cats from catching such bats. In accordance with the observed low contact rate, cats submitted for rabies diagnosis in The Netherlands thus far proved to be EBLV negative.
In The Netherlands, EBLV-1 (genotype 5) seems to be endemic in the Serotine bat and human bite incidents regularly occur. Therefore the public health risk of bat rabies cannot be ignored. More quantitative data on the number of infectious EBLV particles in the saliva of rabid bats are needed. However, our results suggest that, especially in the period between April and October, humans and companion animals in the northern part of the country have a higher risk of encountering rabid bats than those in the southern part.
ACKNOWLEDGEMENTS
We thank Reina van der Heide, Toon Pover, and Betty Verstraten for their technical assistance. This work was financially supported by the Dutch Food and Consumer Product Safety Authority.
DECLARATION OF INTEREST
None.




