Introduction
Cervical carcinogenesis is strongly associated with persistent human papillomavirus (HPV) infection from high-risk human papillomavirus (HR-HPV) [Reference Zur Hausen1]. Its presence is found in 99.7% of cervical cancers worldwide [Reference Walboomers2].
HPV infection is one of the most common sexually transmitted infections in the world, which commonly involves adolescents and young women after sexual debut. As most HPV infections are transient, only a small portion (8–12%) of the HR-HPV-positive patients develop cervical intraepithelial lesions (cervical intraepithelial neoplasia, CIN) and cervical carcinoma (CC). The diagnosis of HPV infection is based on molecular methods that highlight viral nucleic acids, identifying high- and low-risk oncogenic HPV as well as specific viral genotypes. The use of HR-HPV DNA testing could increase the efficacy of cytology for primary cervical cancer screening [Reference Cuzick3]. HPV DNA testing has emerged as a very sensitive screening test that can detect precancerous cervical lesions earlier than cytology.
The discrepancy between younger and older women is because the lesions found in younger women have a higher chance of regression and finding them does not lead to any preventive advantage [Reference Schiffman4].
However, the very high sensitivity of the screening test may also have some negative implications as it may also detect non-progressive cervical lesions. It is very important to predict which patients have an increased risk for cervical lesion progression. It is well established that the viral oncogenes E6 and E7 are responsible for HPV-initiated cervical oncogenesis. HPV E6/E7 oncogene active transcription can be monitored directly through the detection of E6/E7 RNA transcripts or proteins [Reference Cuschieri and Wentzensen5–Reference Cattani7] or indirectly through p16 host protein expression [Reference Benevolo8], affected by the HR-HPV E7 protein and its upregulation.
HPV genotyping, viral load, detection of HPV E6/E7 messenger RNA (mRNA) transcripts and p16 protein expression are markers used to identify all women at higher risk of CIN2 or worse.
Currently, the question of defining the best marker for predicting the progression to cervical cancer is extensively debated in the literature [Reference Keegan9–Reference Waldstrom and Ornskov13]. Additional data and studies are needed to understand better the correlation and clinical significance of these markers.
Histology is widely accepted as the gold standard where a cut-off point of CIN2+ (CIN2, CIN3 or cancer) has been determined as clinically significant and is usually treated; however, it is still unknown which lesions will actually progress to invasive cancer, though some estimates have been made. Based on these observations, it might then be important to assess, in women who show persistence of viral DNA and specific genotype, the expression of E6/E7 mRNA. The objective of this prospective study was to assess whether HPV E6/E7 mRNA positivity in women with atypical squamous cells of undetermined significance (ASCUS) and low-grade squamous intraepithelial lesion (LSIL) at baseline, is able to predict those women who have a high risk of developing a histological CIN2 or worse lesion. We took into consideration the women's age and HPV DNA genotype and followed them up for 3 years.
Materials and methods
Study population
The prospective study was performed at the Colposcopy Outpatient Service of the Gynaecological/Obstetrics Unit at the Policlinico Universitario, Catania (University of Catania, Italy) for secondary screening. The study protocol was approved by the Institutional Review Board of the Department and was conducted in accordance with the 1975 Declaration of Helsinki.
A total of 282 women with positive cervical cytology for ASCUS (77/282) or LSIL (205/282) and HR-HPV infection detected by HPV DNA test were included in the study.
Women were considered eligible for this study if they satisfied the following criteria:
• cervical smear with LSIL confirmed at first colposcopy as CIN1 or ASCUS and persistent in at least two Pap smears;
• not pregnant;
• no evidence of any immunodeficiency;
• no history of therapy for neoplasms;
• HPV-DNA testing positive for HPV 16, 18, 31, 33 and 45.
The average age was 36.6 ± 9.5 years (range 19–59).
Written informed consent was obtained from all participants; all examined patients signed a consent permitting personal data processing.
A cervical smear, colposcopy and DNA HPV test were performed at baseline (enrolment). If patients were HPV DNA positive for at least one of the five HR-HPV types (HPV 16, 18, 31, 33 and 45), the expression of viral oncogenes E6/E7 was investigated.
These women were followed over a 36-month period. A cervical smear, using conventional cytology, and colposcopy were performed every 6 months: at 6 (follow-up 1), 12 (follow-up 2), 18 (follow-up 3), 24 (follow-up 4), 30 (follow-up 5) and 36 (follow-up 6) months. Every 12 months, HPV DNA and mRNA tests were performed. Exo-endocervical cells, collected in ThinPrep solution, were subjected to extraction of total nucleic acids (DNA and RNA) for detecting and genotyping of viral DNA, by means of gene amplification. This was followed by hybridisation with genotype-specific probes able to identify most HPV types of the genital region. The expression of viral oncogenes E6/E7 was investigated, identifying mRNA by the NucliSENS EasyQ HPV assay (bioMérieux).
Analyses of the samples were performed by the Virology Laboratory at the University of Catania, Italy. The colposcopic examination was performed by specialised gynaecologists using an OPM1F Zeiss colposcope (Carl Zeiss, Jena, Germany) and by applying acetic acid and iodine solution Lugol. Colposcopic abnormalities were classified in 3 degrees of increasing severity, according to the nomenclature proposed by the Italian Society of Colposcopy and Cervico-Vaginal Pathology (SICPCV). The visibility or not of the squamous columnar junction was evaluated and biopsies of the cervix and/or endocervical curettage were carried out to guide diagnosis.
A histologic examination was performed on specimens collected by a colposcopy-directed biopsy and/or cone specimens collected by the loop excision procedure.
Our management of CIN1 lesions is to perform a follow-up every 6 months in which women have a new Pap smear; while a colposcopic examination and HPV DNA testing are repeated every 12 months.
All examinations were compared with previous ones to assess the evolution of the lesion.
The primary endpoint was histologically confirmed CIN2+ (CIN2, CIN3) during follow-up.
The women who showed progression to CIN2+ underwent large loop excision of the transformation zone.
HPV infections were classified as a transitory HPV infection, in cases which became HPV negative or exhibited new infections with different HPV types compared with baseline, or a persistent HPV infection if the same genotype as that in the index test was detected.
These women were followed over a 36-month period until detection of CIN2 or worse (progression), HPV infection clearance (clearance) or viral persistence (persistence). Women who exhibited lesion regression at the intermediate examinations completed the scheduled follow-up to ensure that the lesion actually regressed.
HPV testing: NucliSENS EASYQ HPV assay (bioMérieux)
Automated DNA extraction was carried out with a 1 ml sample using the NucliSENS EASYMAG system (bioMérieux SA, Marcy l'Etoile, France) following the manufacturer's HPV 1.1 protocol, with a 55 μl final elution volume.
Amplification of HPV DNA was accomplished by HPV-HS Bio (AB Analiticas.r.l, Padova, Italy) nested polymerase chain reaction (PCR) for the detection of HPV DNA sequences within the L1 open reading frame (ORF), according to the manufacturer's recommendations. To verify the efficiency of the DNA extraction, the housekeeping gene thiosulphate-sulphur transferase (TST) was also amplified. Samples negative for TST were considered inadequate and a new sample was requested.
For the first-amplification step, carried out with 10 µl of eluate, a combination of degenerate primers was used to amplify a 449–458 bp sequence within the L1 ORF of the HPV genome. The second amplification was carried out on 1 µl of the first amplification product, using biotinylated primers to amplify a 139–145 bp sequence. To verify the efficiency of the DNA extraction, 10 μl of eluate were used to amplify a 202 bp fragment of the TST gene using specific primers. Negative (water) and positive controls (plasmid clones containing HPV54) were included for each PCR run to check for accuracy and possible contamination. To confirm amplification, PCR products were submitted to electrophoresis in 3% agarose gel, and the positive ones were used for the hybridisation step. Samples negative for TST were considered inadequate and extracted again from the second tube.
For all the positive samples at a reverse line blot hybridisation assay, HPV typing was carried out with specific probes for the most frequent HPV types (HPV type, AB Analitica s.r.l., Padova, Italy). HPV types allows the identification of 11 LR genotypes (6, 11, 40, 42, 43, 44, 54, 61, 70, 72, 81) and 18 HR genotypes (16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73, 82). Samples that were positive by nested PCR but negative in reverse line blot for any of these types were considered as undetermined HPV.
HPV E6/E7 mRNA testing
The amplification and detection of the E6/E7 mRNA was performed with the NucliSENS EasyQ® HPV kit (bioMérieux, Marcy l'Etoile, France), using PCR with primers/probes for HPV types 16, 18, 31, 33 and 45.
Statistical analysis
Statistical analysis was performed by using the SPSS software package for Windows (version 15.0, SPSS, Chicago, IL, USA). Descriptive statistics were expressed as frequency, arithmetic mean, standard deviation (s.d.) and percentages.
We calculated the relative risk (RR) of women with ASCUS and LSIL and who were E6/E7 mRNA positive to have a progression to CIN2 or worse lesions.
We calculated the cumulative incidence of CIN2+ using the Kaplan–Meier method, for women from enrolment to end of follow-up.
Results
The cohort of 312 HR-HPV positive women had a cytological diagnosis of ASCUS or LSIL at baseline; however, 30 did not complete the follow-up (13 ASCUS and 17 LSIL).
A total of 193/312 (61.8%) ASCUS and LSIL patients were positive for at least one of the five most common types of HPV (16, 18, 31, 33, 45); 61/193 (31.6%) ASCUS, 132/193 (68.4%) LSIL, and had a follow-up of 3 years. The cases of positivity to the DNA of genotypes 16, 18, 31, 33 and 45 were 193/312, for which the NASBA method was subsequently used to search for the E6/E7 mRNA. The most common genotype was HPV16 (n = 124), followed by HPV31 (n = 27), HPV18 (n = 25), HPV45 (n = 9) and HPV33 (n = 8) (Fig. 1).

Fig. 1. Kaplan–Meier curves representing the cumulative incidence of CIN2 in E6/E7 mRNA-positive women.
Table 1 shows the prevalence of the most frequent viral genotypes at baseline. Of these patients, 48.2% (93/193) were positive for genotypes 16, 18, 31, 33 and 45 and were E6/E7 mRNA positive. The HPV E6/E7 mRNA prevalence among the ASCUS and LSIL samples was 50.8% (31/61) and 47% (62/132), respectively (Table 1).
Table 1. Two hundred and eighty-two of 312 patients completed the follow-up period (36 months), 14/31 (45.16%) women in the ASCUS group and 8/62 (12.9%) women in the LSIL group with E6/E7 mRNA-positive developed CIN 2 or worse
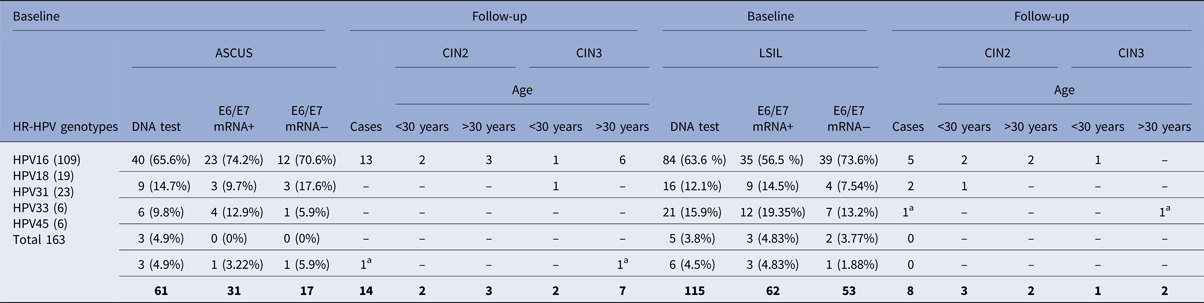
a Only two women (one LSIL and one ASCUS) with E6/E7 mRNA negative developed CIN2 or worse.
Almost all patients completed the follow-up period (36 months), 45.2% (14/31) of the women in the ASCUS group and 12.9% (8/62) of the women in the LSIL group developed CIN2 or worse during the 3 years of follow-up. The corresponding figures for CIN3 at follow-up were 29% (9/31) for the ASCUS group and 4.8% (3/62) for the LSIL group (Table 1). Only 5.9% (1/17) LSIL and 1.9% (1/53) ASCUS with an E6/E7 mRNA-negative test progressed to CIN2.
In this study, all women (22 cases) who developed CIN2 or worse within 3 years of follow-up remained positive for E6/E7 mRNA (Fig. 2). Furthermore, 42.9% (70/163) of women were E6/E7 mRNA negative at baseline and 97.1% (68/70) of these cases did not progress during the follow-up period (i.e. neither CIN2 nor worse).
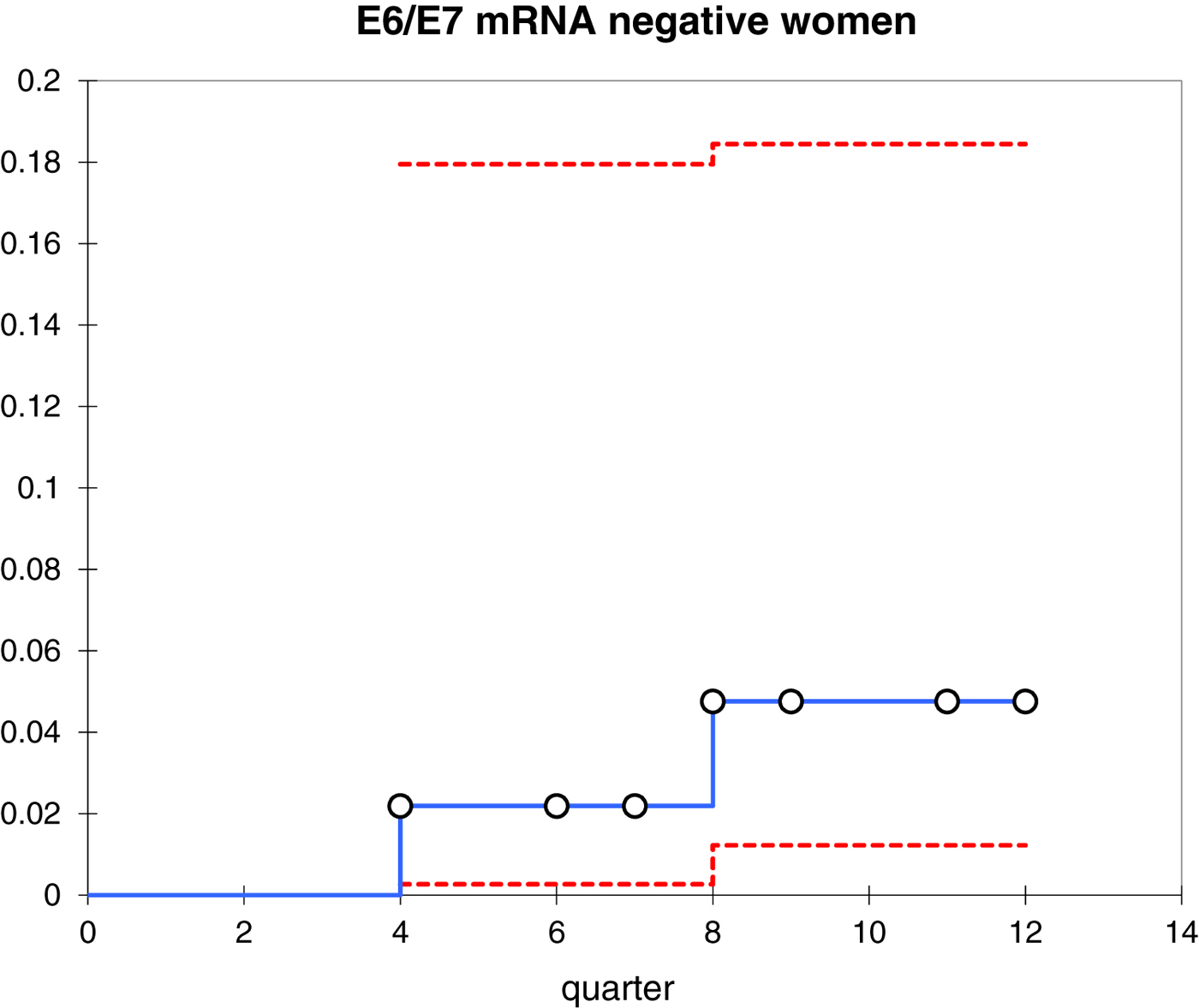
Fig. 2. Kaplan–Meier curves representing the cumulative incidence of CIN2+ in E6/E7 mRNA-negative women.

Fig. 3. Flowchart of the studied population.
Stratified by age, we found that 17/93 women who were positive for mRNA and 30/60 who were mRNA negative were >30 years old; thus, among the patients 30 years of age or older, we found an incidence of 14/22 (63.6%) of CIN2+ lesions, with 64.3% (9/14) CIN3 (RR = 8.7, 95% CI 2.1–35.9).
The results of RR for progression vs. CIN2 or worse in E6/E7 mRNA-positive women with LSIL, ASCUS and HPV16 is shown in Table 2.
Table 2. Relative risk (RR) for progression vs CIN2+ in E6/E7 mRNA-positive women with LSIL and ASCUS lesions

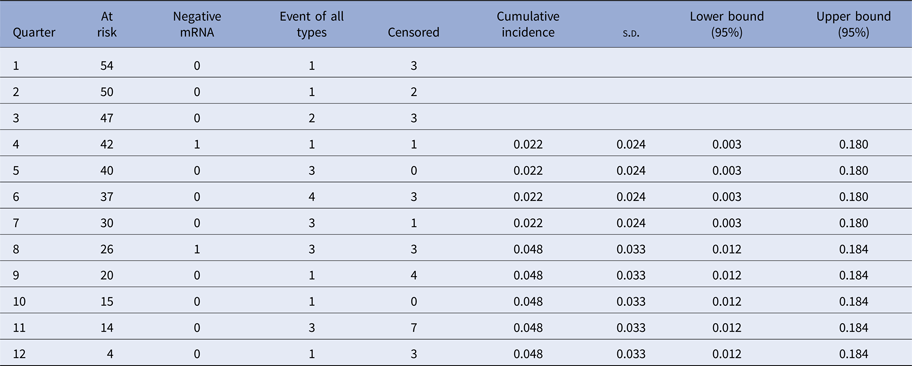
The Kaplan–Meier curves (Fig. 1) show the cumulative incidence of CIN2 in E6/E7 mRNA-positive women at 12, 24 and 36 months which was 0.079 (95% CI 0.031–0.203), 0.362 (95% CI 0.243–0.538) and 0.644 (95% CI 0.459–0.904), respectively (Tables 3 and 4).
Table 3. Cumulative incidence of CIN2+ in E6/E7 mRNA-positive women
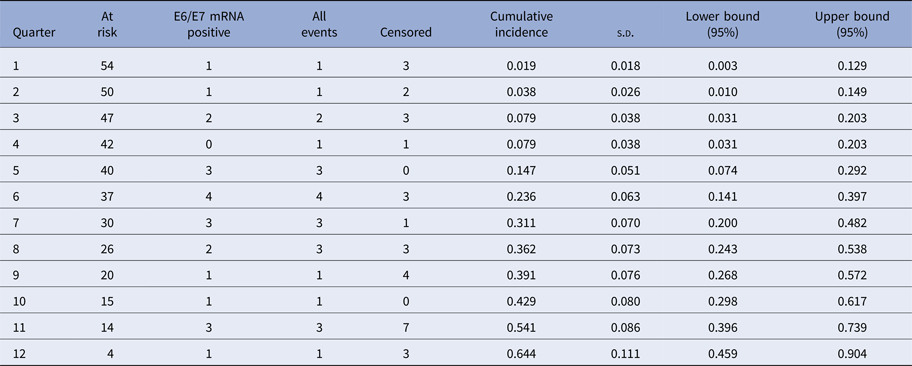
Table 4. Cumulative incidence of CIN2+ in HPV E6/E7 mRNA-negative women
All women with histological CIN3 were treated by cervical conisation and cone-biopsy histology was carried out. They were all confirmed to be CIN3 in the cone. The results are shown in Fig. 3.
Discussion
The HPV test in Italy is used to triage women with ASCUS and LSIL cytological cervical lesions.
In triage, it is important to have a test with high specificity; in fact, the use of an HPV DNA test that has high sensitivity and low specificity referred a high number of women to an unnecessary colposcopy and biopsy or cervical conisation, especially when applied to younger women.
A marker of oncogenic activity could be the presence of HPV mRNA transcripts coding for E6/E7 proteins, since the expression of these genes inactivates p53 and pRb tumour suppressors (retinoblastoma), respectively [Reference Yugawa and Kiyono14]. Furthermore, the expression of E6/E7 oncoproteins seems mandatory for HPV-induced cellular immortalisation, transformation and tumour progression; consequently, a test that detects the overexpression of E6/E7 mRNA is more specific than a test that detects the general presence of viral DNA. In this context, HPV E6/E7 mRNA expression might be predictive of disease progression and might constitute a useful tool for screening or patient management.
Molden et al. [Reference Molden15] in a study that involved 77 women showed that E6/E7 mRNA is more efficient than DNA testing for the prediction of CIN2 or worse (84.8% and 50%, respectively).
Currently, three commercial tests exist for detecting HPV E6/E7 mRNA. The PreTect HPV-Proofer (NorChip) and the NucliSENS EasyQ HPV (bioMérieux) are based on the same technology; however, they are marketed under different brand names in different countries. The PreTect HPV-Proofer and NucliSENS EasyQ detect E6/E7 mRNA expression from the five most prevalent HR-HPV types (16, 18, 31, 33 and 45). The APTIMA HPV Assay (Gen-Probe) targets mRNA expression from the 14 most carcinogenic HR-HPV types.
In our diagnostic practice in the triage of women with minor cytological cervical lesions, we use NucliSENS EasyQ HPV (bioMérieux) for detecting HPV E6/E7 mRNA.
Our prospective study investigated the diagnosis of histological CIN2 or worse in women with cytological ASCUS or LSIL at baseline and with E6/E7 mRNA positive over a 3-year follow-up.
HPV status was one of the most significant determinants of developing high-grade cervical disease during follow-up. HPV16 is the most frequent HPV genotype among women with high-grade cervical lesions and women with histologically confirmed CIN2 or worse.
The rather high risk of developing CIN2 or worse for minor cytological lesions (LSIL, ASCUS) reflects the high prevalence of HPV16 (79.4%) in this diagnostic category. As in our study, several others previously demonstrated a high-risk for pre-cancer among HPV positives and especially among women positive for HPV16 and/or HPV18. Women infected with HPV16 and HPV18 are considered particularly at high risk and these types account for approximately 70% of CC worldwide.
The most common HPV genotypes detected in most previous European studies were HPV16 and HPV31, and in the USA, they were HPV16 and HPV45, whereas the present study showed the most prevalent genotypes in our patients to be HPV16 and HPV31, in agreement with other European studies [Reference Clifford16].
Cuschieri et al. [Reference Cuschieri, Whitley and Cubie17] carried out a 2-year follow-up of cytologically normal women with repeated HPV genotyping using both PCR and PreTect HPV-Proofer. They reported that detection of E6/E7 transcripts was less sensitive but more specific than detection of HPV DNA for the detection of disease at follow-up.
Women who were positive for HPV DNA and mRNA transcripts at baseline were significantly more likely to harbour persistent infection compared with those women in whom only DNA was detected at baseline.
In our study, 36.3% (70/193) of patients were mRNA negative at baseline and 97.1% (68/70) of the cases were non-progressors during the follow-up period (i.e. neither CIN2 nor worse).
Cox et al. [Reference Cox, Schiffman and Solomon18] concluded that HPV-positive LSIL and HPV-positive ASCUS women are clinically equivalent and that the cumulative risk of CIN2+ was equivalent for HPV-positive LSIL women (27.6%) and HPV-positive ASCUS women (26.7%).
In our research, a similar observation regarding the clinical equivalence of LSIL and ASCUS HPV-positive women was observed (Table 2).
Our prospective study investigated the RR for diagnosis of histological CIN2 or worse (Table 2) in women with HPV mRNA positive. We detected that a woman having an ASCUS/LSIL Pap smear and a E6/E7 mRNA-positive result was 6.9 (95% CI 1.7–28.8) times more likely to be diagnosed with CIN2+ than a woman with HPV mRNA-negative result. Furthermore, 50.5% (47/93) of the E6/E7 mRNA-positive women were older than 30 years of age with an RR of 8.7 (95% CI 2.1–35.95) for CIN2+ diagnosis.
The small sample size and the relatively short follow-up period are limitations for independent conclusions.
Therefore, the identikit of the woman at risk for progression towards a CIN2+ lesion becomes clearer: women with persistent HPV16 infection, E6/E7 mRNA positive and older than 30 years of age. These women should undergo a more detailed follow-up, those who are HPV E6/E7 mRNA negative may have a less frequent follow-up.
The massive presence of HPV infection in the female population, often unrelated to the presence of progressive cytologic lesions over time, as presented in this study, justifies the implementation of HPV E6/E7 mRNA as a more specific test, with respect to DNA testing, for first-line screening of cervical cancer and its use in screening programmes. The major benefit of this study is to identify patients at high risk of progression so as to avoid subjecting women at low risk to continuous examinations, avoiding patient anxiety with a consequent improvement in cost/benefits, as well as the possibility of using the mRNA test in post-negative colposcopy follow-up.
Acknowledgements
The authors thank the Scientific Bureau of the University of Catania for language support.









