INTRODUCTION
Ross River virus (RRV) is the causal pathogen of the most widely spread mosquito-borne disease (MBD) in Australia. Thousands of clinical cases are reported to the Australian Department of Health and Ageing each year. In 2011, RRV infections accounted for 60·3% (n = 5139) of all reported MBD notifications [1]. Although outbreaks, epidemics, small case clusters, and incidental cases have been reported from all Australian states and territories each year (Fig. 1), most notifications were from Queensland, tropical Western Australia and the Northern Territory [1]. For example, from 1993 to 2012, the notifications from Queensland accounted for an average of 49% of the total cases, ranging from 78% in 1994 to 42% in 2012. Between 1993 and 2012 the average incidence for Queensland was 57/100 000, compared to the national average of 22/100 000 (compiled from communicable diseases data http://www9.health.gov.au/cda/Source/CDA-index.cfm) [1].

Fig. 1 [colour online]. Number of notifications of Ross River virus infection, received from State and Territory health authorities, 1993–2012. CT, Capital Territory; NSW, New South Wales; NT, Northern Territory; Qld, Queensland; SA, South Australia; Tas, Tasmania; Vic, Victoria; WA, West Australia. (Source: Australian Department of Health and Ageing. National Notifiable Diseases Surveillance System: http://www9.health.gov.au/cda/source/Rpt_4.cfm).
RRV outbreaks have increased throughout the country, notably in urban settlements and tourist destinations [Reference Harley, Sleigh and Ritchie2–Reference Werner6]. Over the past decade, Queensland Health has noted an increase in disease incidence throughout the state, including expansion into areas where the disease was previously absent, possibly related to an increase in travel [Reference Dwyer7]. The annual costs of symptom management and productivity losses are estimated at A$3–6 million, although this sum does not account for public health surveillance, control and response activities or full diagnostic and medical costs [Reference Harley, Ritchie, Bain and Sleigh3, Reference Hawkes8]. The cost of healthcare resources and productivity loss have been estimated at A$1000–2500 per person between 1996 and 2002 [Reference Harley, Ritchie, Bain and Sleigh3, Reference Boughton9, Reference Mylonas, Brown and Catthew10], while it was more recently estimated to be over A$1000 per person (varying from A$1018 to A$1180) [Reference Ratnayake11, Reference Woodruff and Bambrik12] and the aggregated cost was A$4.3–4·9 million in 2007 [Reference Woodruff and Bambrik12]. Outbreaks of RRV impact considerably on tourism and industry, as well as on local communities [Reference Tong, Hu and McMichael5, Reference Tong and Hu13].
The epidemiology of RRV is complex because transmission cycles are driven by various mosquito species and vertebrate hosts within a variety of disparate geoclimatic regions [Reference Kelly-Hope, Purdie and Kay14–Reference Russell16]. More than 40 mosquito species have been implicated as vectors of RRV [Reference Russell16, Reference Mackenzie17]. Significant vectors include, Aedes vigilax and A. camptorhynchus with larval habitats in inter-tidal coastal wetlands, other Aedes species breed in fresh water and are found in many inland areas, and Culex annulirostris, which is found in vegetated semi-permanent and permanent fresh water, is common in tropical and temperate areas. Marsupials (e.g. kangaroos, wallabies) and other animals (e.g. dogs, cats, horses, possums) are implicated as intermediate vertebrate hosts for this disease. The incubation period may be as short as 3 days or as long as 21 days (usually 7–9 days on average) [Reference Harley18].
Mosquitoes are cold-blooded (ectothermic) and thus are especially sensitive to climatic changes [Reference McMichael, Woodruff and Hales19]. RRV is likely to be affected by climate change because weather influences the survival and reproduction rates of mosquitoes. This in turn influences distribution, abundance, intensity and temporal patterns of mosquito activity (particularly biting rates) throughout the year; as well as rates of development, survival and reproduction of pathogens within mosquitoes [Reference Rogers and Randolph20]. These results suggest that RRV may become increasingly widespread in the future due to global warming [Reference Costello21].
There is no vaccination or specific treatment for RRV infection. The most effective way to prevent infection is to protect the person from mosquito bites. As climate conditions can directly and indirectly affect mosquito density and mosquito habitats, there is growing concern regarding the future risks of arbovirus diseases arising from climate change [Reference Werner6, Reference Hu22, Reference Tong23]. Therefore, it is vitally important to make appropriate projections on the future spread of RRV under various climate change scenarios in order for policy-makers to identify vulnerable communities and better manage RRV epidemics and mosquito vectors.
This paper brings together and critically reviews the current methodological challenges and research needs for projecting the potential impacts of climate change on RRV, which may have wide applications in the development of effective risk management programmes for RRV and other MBDs nationally and internationally.
METHODS
A literature search was conducted and updated between January and October 2012, using the electronic databases Medline, Web of Science and PubMed. The key words and Medical Subject Headings (MeSH) terms used were: ‘climate’, ‘climate change’, ‘climate variability’, ‘global warming’, ‘greenhouse effect’ AND ‘Ross River virus’, AND ‘project*’, ‘forecasting’, ‘future’ or ‘scenario*’. References and citations of the articles identified were inspected to ensure that the relevant articles were included in the detailed review.
Two inclusion criteria were used to select articles. First, only quantitative, empirical studies published in peer-reviewed English-language journals were selected. Second, a paper had to contain information on the possible impact of climate change on RRV disease.
RESULTS
General
We identified 42 papers from searches of the selected electronic bibliographical databases. After reviewing the titles and abstracts of these papers, we retrieved 26 articles for the detailed examination. Finally, 19 studies met the eligibility criteria and were included in the review. Figure 2 shows a flowchart of this process and the reasons for exclusion of articles at each stage.

Fig. 2. Flowchart of literature search.
Among the relevant papers identified, only two studies projected how changes in rainfall patterns will affect RRV disease in Australia by 2100 using ‘dry season’ and ‘wet season’ RRV scenarios [Reference Woodruff and Bambrik12, Reference Bambrick and Woodruff24]. The results presented in both studies were similar. Changes to transmission patterns are thought to be regionally specific. The annual pattern will probably change from epidemic to endemic in temperate regions that border sub-tropical endemic regions. An increase in months of RRV activity may occur in cooler temperate regions such as southern Tasmania, higher altitude New South Wales and Victoria. Marked decreases in rainfall in southwest Western Australia may reduce RRV in this location. However, long drought periods may provide suitable conditions for building numbers of susceptible kangaroo hosts, which suggests a stronger pattern of large outbreaks of RRV disease interspersed by years of inactivity in certain temperate and semi-arid regions [Reference Woodruff and Bambrik12]. In coastal areas, salt-water vector breeding will be enhanced by increased tidal inundation due to sea-level rise and population growth will increase the number of people at risk of infection. Because of the increased scarcity and cost of water leading to reduced irrigation, the irrigation areas of southwest New South Wales and northern Victoria will cease to be a significant location of RRV activity [Reference Woodruff and Bambrik12].
Seventeen other studies developed the predictive early warning models and assessed the relative risks of climate, mosquito and other socioeconomic factors on RRV, based on retrospective data which explored the model validation, inclusive variables and regional variations (Table 1). In general, the studies cover all Australian climates, except equatorial and desert (Fig. 3). The body of research covered a range of areas including Queensland [Reference Tong, Hu and McMichael5, Reference Tong and Hu13, Reference Hu22, Reference Bi and Parton25–Reference Tong32], Victoria [Reference Woodruff33], Western Australia [Reference McIver4, Reference Woodruff34], South Australia [Reference Bi35, Reference Williams, Fricker and Kokkinn36], Tasmania [Reference Werner6] and the Top End of the Northern Territory [Reference Jacups37].

Fig. 3 [colour online]. The climates of the included study areas.
Table 1. The characteristics of the included studies
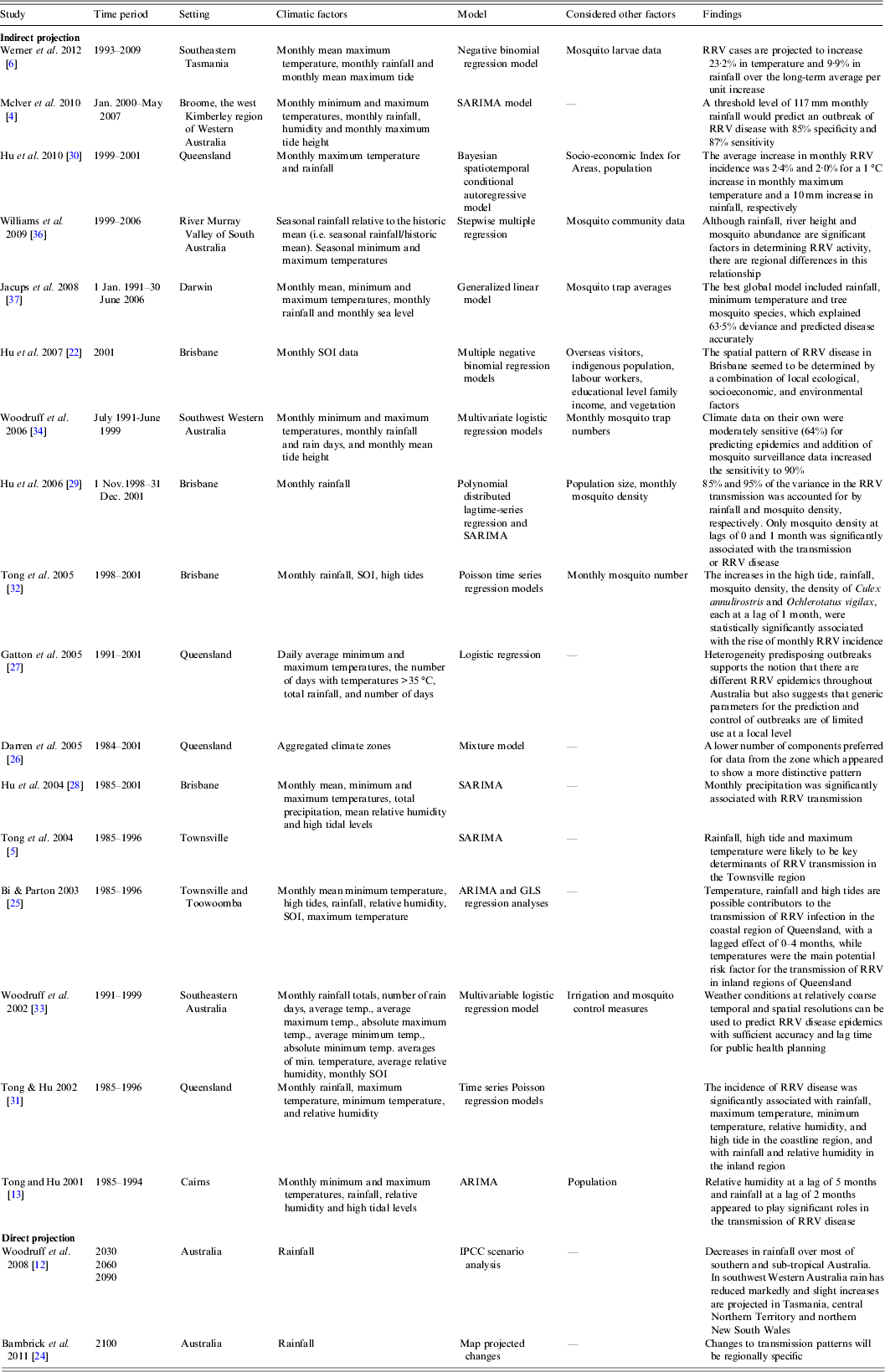
ARIMA, Autoregressive integrated moving average; GLS, generalized least square; IPCC, Intergovernmental Panel on Climate Change; RRV, Ross River virus; SARIMA, seasonal autoregressive integrated moving average; SOI, Southern Oscillation Index.
These studies appear to confirm that several environmental variables, including rainfall, temperature, humidity, and high tide (or even river height as demonstrated in the article by Williams et al. [Reference Williams, Fricker and Kokkinn36]) are correlated with mosquito abundance and subsequent RRV activity. Several of these studies demonstrate that the environmental variables related to RRV disease incidence often differ between regions, thus making it difficult to generalize findings from one regional model to another [Reference Harley, Sleigh and Ritchie2, Reference Gatton, Kay and Ryan27, Reference Tong and Hu31, Reference Woodruff33, Reference Williams, Fricker and Kokkinn36]. A lagged effect of climate drivers on RRV transmission has also been identified in these studies. Lags have been employed to account for virus/mosquito lifecycle, incubation period in the human host, and presentation to a medical practitioner [Reference Jacups, Whelan and Currie38]. Combining mosquito population data with locally available and easily accessible climate data has been shown to enhance the ability of local models to predict RRV disease outbreaks [Reference Woodruff33, Reference Jacups37]. Other socio-ecological factors were also important contributors in studying the climate–RRV relationship [Reference Harley, Sleigh and Ritchie2, Reference Tong23, Reference Woodruff33].
There were generally three steps in the published research to assess the pre-existing association between RRV and climate. First, the model was developed using various independent variables with RRV as the dependent variable. Various models were used in the included articles. The two main models were seasonal auto-regressive integrated moving average (SARIMA) and auto-regressive integrated moving average (ARIMA) used in six of the studies, various regression models were used in seven studies and four others [Intergovernmental Panel on Climate Change (IPCC) scenario analysis, change mapping, mixed models, generalized linear models (see Table 1)]. Some studies used more than one model to compare the results [Reference Bi and Parton25, Reference Hu29]. Second, model fit was checked. Finally, the model was verified by predictive variables matched to the actual observations. The criteria for choosing a model included Bayesian Information Criterion [Reference Jacups37], Akaike's Information Criterion (AIC) [Reference Hu28], change in AIC (ΔAIC) [Reference Jacups37], goodness-of-fit test [Reference Tong, Hu and McMichael5, Reference Gatton, Kay and Ryan27, Reference Hu29, Reference Woodruff33, Reference Woodruff34], and Deviance Information Criterion [Reference Darren, Zerger and Argent26, Reference Hu30].
The impact of important climate variables on RRV transmission
Rainfall has almost universally been included in models of RRV infection around Australia [Reference Jacups, Whelan and Currie38]. As shown in a Brisbane study, 85% of the variation in RRV incidence was accounted for by rainfall [Reference Harley, Sleigh and Ritchie2]. In Broome, 117 mm of monthly rainfall was associated with a 95% likelihood of an outbreak of RRV disease, with 85% specificity and 87% sensitivity [Reference McIver4]. For a lag period of 0–4 months, Tong & Hu found no significant correlation between rainfall and RRV incidence in Gladstone [Reference Tong and Hu31]. A significant positive correlation was evident during a 1-month lag in Darwin [Reference Jacups37] and South Australia [Reference Bi35], a 2-month lag in Brisbane [Reference Hu28, Reference Tong and Hu31], Townsville [Reference Tong, Hu and McMichael5] and Cairns [Reference Tong and Hu13] and the whole of Queensland [Reference Tong and Hu31], a 3-month lag in Mackay [Reference Tong and Hu31] and Tasmania [Reference Werner6], and a 4-month lag in Bundaberg [Reference Tong and Hu31]. Some studies showed a significant correlation between rainfall and vectors, during a 1-month lag in Brisbane [Reference Tong32] and Darwin [Reference Jacups37] for Cx annulirostris and A. vigilax vectors, as well as 2- and 4-month lag times in Townsville and Toowoomba for A. vigilax [Reference Bi and Parton25], and a 2-month lag in Darwin for A. notoscriptus [Reference Jacups37].
Other climatic indicators seemed to play a role in the transmission of RRV disease but the effect was region-specific. Minimum temperature was positively associated with RRV during a 1-month lag in South Australia [Reference Bi35], a 2-month lag in Townsville [Reference Tong, Hu and McMichael5] and Queensland [Reference Tong and Hu31] and a 3-month lag in Darwin [Reference Jacups37]. A positive relationship was found between maximum temperature and RRV infection in Townsville [Reference Tong, Hu and McMichael5], Queensland [Reference Bi and Parton25, Reference Tong and Hu31] and Tasmania [Reference Werner6]. The Southern Oscillation Index (SOI) had a positive association during a 2-month lag in South Australia [Reference Bi35] and Brisbane [Reference Harley, Sleigh and Ritchie2]. Humidity was negatively related to the number of cases in the previous month in South Australia [Reference Bi35], a 2-month lag in Townsville [Reference Tong, Hu and McMichael5] and a 5-month lag in Cairns [Reference Tong and Hu13].
High tide was implicated as an important precursor of RRV outbreaks in several studies. The negative relationship between tide and RRV cases at 0- and 1-month lag times in Tasmania is novel to the study by Werner et al. [Reference Werner6]. A positive relationship between maximum tide and RRV infections in the current month was found in Darwin [Reference Jacups37] and Townsville [Reference Tong, Hu and McMichael5]. Absolute tide height was positively related to RRV in Bunbury [Reference Woodruff34].
Climate data alone was moderately sensitive (e.g. 64%) in predicting epidemics and the addition of mosquito surveillance data increased the sensitivity of the early warning model to 90% in Western Australia [Reference Woodruff33, Reference Woodruff34]. The best model was found to include climate indicators and mosquito species, which explained more deviance than climate-only or vector-only models [Reference Jacups37]. A study in Brisbane found that 95% of variation in RRV incidence was accounted for by mosquito density [Reference Hu29]. While not demonstrating causative relationships, several mosquito species were significantly associated with increased virus activity. In the Renmark-Paringa Local Government Area (LGA), Coquillettidia linealis, known to be an efficient vector of RRV, was found to be a significant factor. Conversely, time-lagged Cx globocoxitus abundance was also found to be a significant predictor of activity, despite not being considered a competent vector of RRV [Reference Williams, Fricker and Kokkinn36].
Climate is not the only factor affecting RRV incidence. Other socio-ecological factors were also important contributors in studying climate–RRV relationships [Reference Hu22, Reference Tong23, Reference Woodruff33]. For example, there was a positive relationship between RRV incidence and the proportion of people with lower levels of education and with vegetation density. There was a negative relationship between RRV incidence and the proportion of labour workers. A decrease in RRV incidence was related to an increase in the proportion of labour workers. [Reference Hu22]. However, non-climatic factors are seldom included in much of the research cited, possibly due to data availability. No models to date have incorporated data on vertebrate hosts or information on human population movements from non-endemic areas to endemic areas [Reference Jacups, Whelan and Currie38].
DISCUSSION
Climate change is likely to increase the risk of RRV transmission
The fourth assessment report of the IPCC reported that global mean surface temperature will probably rise by between 1·1°C and 6·4°C by 2100, with best estimates of between 1·8°C and 4·0°C. During this century, global average surface temperature increases are likely to exceed the safe threshold of 2·0°C above the pre-industrial average temperature [39]. In Australia, if emissions are low, warming of between 1°C and 2·5°C is likely to occur by around 2070, with a best estimate of 1·8°C. Under a high emission scenario, the best estimate for warming is 3·4°C, with a range of 2·2°C to 5°C [40]. It also predicts that decreases in annual average rainfall are likely to occur in southern Australia, droughts are likely to become more frequent and sea levels will continue to rise [40]. These changes will increase the level of climate change-related risks for the rest of the century and create significant challenges in terms of RRV infection and endemicity [Reference Costello21, Reference Tong23].
Rainfall is considered the most important climatic factor driving RRV prevalence due to mosquitoes' reliance on water to complete their lifecycle [Reference Tong, Hu and McMichael5, Reference Werner6, Reference Hu28]. At higher temperatures but before reaching the upper threshold, the proliferation and reproduction rates increased, the transmission season was extended, and ecological balances and climate-related migration of vectors, reservoir hosts, or human populations changed [Reference Semenza and Menne41]. Tidal inundation of salt-marshes is a major source of water for breeding of the important arbovirus vectors A. vigilax and A. camptorhynchus. Adult females of both species lay their eggs on soil, mud substrate and the plants around the margins of their larval habitats. The eggs hatch when high tides subsequently inundate the sites. Large populations of adult mosquitoes can emerge as soon as 8 days after a series of spring tides [Reference Tong, Hu and McMichael5].
Because RRV is transmitted by various mosquito species in different situations, the epidemiology of the disease varies between and within regions. The different risk factors identified in the different regions possibly reflect the underlying climatic environments, and/or different vector populations and habitats [Reference Gatton, Kay and Ryan27]. In the temperate south, epidemic activity is usually associated with summer and autumn rainfall in inland areas or rain and/or tidal inundation of coastal marshes during the warmer seasons, when the vectors are most active. In the tropical north, distinct seasonal activity associated with the highest spring tides and the wet season is apparent, and in some regions there may be year-round activity [Reference Russell15]. Many cases of infections occur in coastal regions which have inter-tidal salt-marsh and mangrove habitats harbouring large populations of mosquitoes that can carry the virus. However, cases are also reported in inland areas of Australia, in response to rainfall and salinity. In northern and central Queensland, RRV is active throughout the year, in other states, disease presence follows spring and summer rains.
Projection is a useful tool for policy-making
RRV is one of the few infectious diseases that can be predicted by climate-based early warning systems [Reference Woodruff34]. Early warning of weather conditions conducive to outbreaks of RRV disease is possible at the regional level, with a high degree of accuracy [Reference Woodruff33]. It is important to make appropriate projections on the future spread of RRV under various climate change scenarios because such information is essential for policy-makers to identify vulnerable communities and to better manage RRV epidemics [Reference Woodruff and Bambrik12, Reference Sutherst42]. Using climatic indices along with forecasting models can alert authorities to possible changes in the risk level, either immediately or in the near future [Reference Bulto43]. The ability to predict an outbreak months or years in advance based upon climatic indicators may make it possible to implement early intervention initiatives or aggressive vector control programmes [Reference Patz and Khaliq44].
Current challenges
There have been several key methodological challenges in projecting the impact of climate change on RRV transmission from the projection research of climate change MBDs. These include:
-
(1) Data selected are heavily dependent on availability and technique limitation. For example, climate data in a LGA is generally sourced from the station with the most complete data or the longest recording history [Reference Gatton, Kay and Ryan27, Reference Hu28].
-
(2) Analytical scales (e.g. monthly) may be too coarse to assess the immediate effects of climate variation on RRV disease; however, the incidences of RRV may be too low to assess if weekly or daily indices are used [Reference Hu28].
-
(3) A number of IPCC emission scenarios were applied and various baseline time periods were chosen in previous projections of climate change on other MBDs. No standard method is recommended.
-
(4) In order to project future climate factors within specific regions, downscaling techniques and regional models need to be applied to project future regional climate change. However, regional climate models are very complex and there are no consistent procedures.
-
(5) Both biological and statistical models were used to describe the climate–MBD relationship and the modelling technique is not yet standard. In addition, there are limitations to these models in that potential changes to the immune status of the human population, and in the ecology of vertebrate virus reservoirs, have not been taken into account [Reference Williams, Fricker and Kokkinn36].
-
(6) Finally, uncertainties exist in scenario-based climate change risk assessment. Future greenhouse gas emissions, population growth, socioeconomic status, urbanization, land-use change phenomena, migration and disease control programmes will affect future risks of RRV outbreaks.
Recommendations for future research directions
An assessment of the future risk of RRV transmission associated with climate change will ultimately need to integrate global and regional climate-based analysis with local socioeconomic and environmental factors, in order to guide comprehensive and sustainable strategies to control and prevent RRV transmission. We therefore make the following recommendations for future research directions:
-
(1) The availability of climate data at a sub-regional level [e.g. Statistical Local Area (SLA) or LGA] over a long-term period will assist better understanding of the distribution of RRV transmission across the country. Accordingly, the climate data at various stations at a sub-regional level should be incorporated in the models to project the distribution of the disease in both time and space.
-
(2) Improved understanding of RRV ecology, and the direct and indirect effects of climate variability on RRV cycles, is required to establish the baseline relationship between climatic factors and RRV transmission.
-
(3) A local model (e.g. SLA/LGA level) for projecting future climate change-related RRV risk needs to be developed. This is an area which is relevant not only for developing adaptation strategies to the direct climate change effects on RRV but also for planning interventions to reduce the indirect effects of factors such as land use and ecological degradation. In particular, the development of combining process-based models (capturing the ecology of the RRV transmission system) with a statistical approach is needed.
-
(4) Uncertainty analysis should be included within the model. The problem with non-climatic drivers of RRV transmission is that most of their impacts have not been quantified and thus, minimizing these uncertainties would markedly improve the outcomes of future projections of climate change impacts on RRV.
-
(5) For any predictive models to become part of effective warning systems, they must provide reliable forecasts with clear parameters for triggering response activities. The specificity of the models for particular regions means that they are limited in geographical applicability. Such models are most effective when developed for specific regions [Reference Williams, Fricker and Kokkinn36].
CONCLUSION
Future risk assessments of climate change will ultimately need to better understand the ecology of RRV disease and to integrate climate change scenarios with local socioeconomic and environmental factors, in order to develop effective adaptation strategies to control and prevent RRV transmission. Current methods of projecting the impact of climate on RRV transmission are still at an early stage and need further development.
DECLARATION OF INTEREST
None.







