INTRODUCTION
Although a marked reduction in incidence of measles have been observed in the last decade, recently its re-introduction and outbreaks have re-emerged in Turkey, as in many other parts of the world [1]. Suboptimal vaccination coverage is thought to be the main cause of this situation in Europe but in contrast, an outbreak of measles occurred despite high coverage of measles vaccination (over 95%) in Turkey. There were no more than a few cases anually between 2007 and 2010, but hundreds of cases began to occur in 2011–2012 and thousands of cases were seen in 2013. Increased incidence of measles after immigration with cases mostly reported from the south-eastern part of the country near the border have suggested the possible influence of refugees from Syria.
Patients in hospitals face the risk of becoming infected during a measles outbreak in the community. There were several outbreaks that could not be controlled which affected hundreds of individuals in hospitals [Reference Lee2–Reference Green4]. When an outbreak of measles occurs in the hospital setting, the consequences can be severe and the cost substantial [Reference Botelho-Nevers5]. Therefore establishing a definitive diagnosis, reporting the case, identifying susceptible individuals and implementing prevention measures are the cornerstones of prevention [Reference Maltezou and Wicker6].
PATIENTS AND METHODS
Gazi University Hospital is a 15-floor, 1000-bed tertiary hospital in the capital city of Turkey, Ankara. Our general paediatric ward is an open, 51-bed ward, with two combined corridors with 25 and 26 beds in each, with physical walls between some of the bed spaces, and lacking a negative pressure room. The mother of a child with methylmalonic acidaemia presented with fever and generalized maculopapular erythematous rash following non-specific coryzal symptoms on her child's third day of hospitalization. The ward was on alert status due to the suspicion of measles. It was learned that the mother had sustained interaction with all of the parents on the ward and had spent considerable time in the corridors of the ward before the onset of her rash. She was quickly masked and taken to an adult infectious disease service, where she was isolated in a separate room lacking negative pressure and was discharged from hospital the following day. Her 5-year-old child had no clinical finding of measles but had an unexplained fever, the child was taken to a paediatric infectious disease service and was isolated in a separate room lacking negative pressure. The mother was diagnosed as having measles by confirmatory serology at the reference laboratory of Refik Saydam Hifzissiha, Ankara. She was reported immediately to the local health department. Fortunately, finding cases who might have been exposed did not require exhaustive investigation because only parents are allowed to visit at the child's bedside in accordance with hospital policy.
Exposed persons without evidence of immunity [i.e. no documentary evidence of receiving two doses of measles-mumps-rubella (MMR) vaccine] were treated with intravenous immunoglobulin (IVIG) or vaccination. The decision to treat was made according to the patient's age, underlying disease, and immune status. Employees unable to provide documentary evidence of receiving two doses of MMR vaccine were vaccinated promptly but could not be excluded from the workplace. The interruption of transmission with IVIG and vaccination was evaluated. Follow-up of the exposed parents and children who were discharged from the hospital was done by telephone.
RESULTS
There were 44 children and 101 adults exposed to the index patient. Twenty-five children and 88 adults providing documentary evidence of receiving two doses of MMR vaccine were considered totally immune. Nineteen children and 13 adults lacking documentary evidence of immunity were either given IVIG or vaccination.
Of the exposed 44 children, nine had received 400 mg/kg IVIG, 10 had been vaccinated with MMR and 25 fully immunized children did not receive either. Only the child of the index patient had serology and measles IgM and IgG were both negative despite the child receiving one dose of MMR vaccine at age 1 year. She was given IVIG but not MMR vaccine for prophylaxis because of her underlying condition and existence of fever. Her fever resolved within 3 days. No cause of fever could be established after a detailed examination. No rash appeared on her follow-up at 3 weeks. The second dose of her MMR vaccine was planned to be given after a 5- to 6-month period. Detailed information of children receiving post-exposure prophylaxis (PEP) with IVIG is given in Table 1. The vaccination schedule of the children who received IVIG was planned to be completed according to their underlying condition.
Table 1. Characteristics of children who were given IVIG for post-exposure prophylaxis
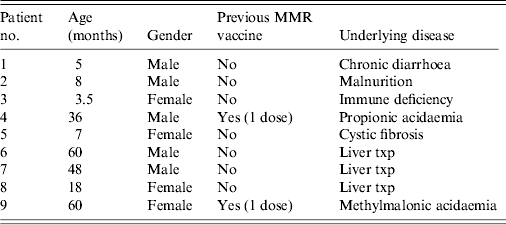
IVIG, Intravenous immunoglobulin; MMR, measles-mumps-rubella.
Four of the 10 children who were vaccinated were aged between 6 and 9 months and had not been vaccinated previously. The other six children who were vaccinated had received one dose of MMR vaccine. Thirteen employees unable to provide documentary evidence of immunity for measles were vaccinated promptly without screening by serology due to the outbreak status. None of the parents required vaccination as they were able to provide evidence of immunity. After 21 days follow-up, no subsequent spread of measles occurred either in children or in adults who had been exposed.
DISCUSSION
Despite a marked reduction of the related morbidity and mortality globally, measles continues to circulate woldwide [Reference Ostroff7]. Hospital-acquired transmission is an important mode of transmission and when a case of measles is suspected in a healthcare facility, minimizing the potential for transmission requires extensive investigation. PEP, tracking contacts, exclusion and case isolation are all indispensable measures of prevention policies. However, when such measures are unable to be implemented, vaccination can be the only effective way of preventing the spread of measles. Several studies have demonstrated that measles vaccine is effective in preventing the development of clinical measles in exposed individuals if vaccination is performed within 72 h of exposure [Reference Pickering8]. The rate of protection varied between 68% and 100% [Reference Berkovich and Starr9, Reference Ruuskanen, Salmi and Halonen10]. Timing of vaccination is important for the rate of protection. Rapid diagnosis and awareness of disease are the important issues at this point. Vaccination of 10 exposed children and 13 exposed employees within 24 h provided 100% success rate in our facility. We vaccinated 13 employees who had been exposed and were not able to provide documentary evidence of measles immunity promptly instead of screening with serology; none of these individuals developed measles. Vaccination should not be delayed while waiting for serological results during an outbreak.
Exclusion of all susceptible exposed persons from the workplace is not mandatory, except in a high-risk setting, if vaccination is administered within 72 h of exposure; however, current guidelines suggest exclusion [Reference Bolyard11]. It is recommended that healthcare personnel without evidence of immunity who have been exposed should be relieved of direct patient contact from days 5 to 21 after exposure even if they have received vaccination. We could not exclude vaccinated employees because of insufficient staff numbers but observed them closely for the development of prodromal illness consistent with measles. None of these individuals developed any symptoms after PEP with MMR vaccine.
Since measles is highly contagious in a healthcare setting, isolation of potential patients is crucial to control the outbreak. When a case of measles is suspected in a healthcare facility, initiation of respiratory isolation is recommended. Private rooms with negative-pressure air ventilation and the use of masks are required. However, such private rooms are not always avaible in many countries. Although strict respiratory isolation techniques have been demonstrated to decrease transmission rates in an outbreak [Reference Raad12] we were unable to provide respiratory isolation in a negative pressure room. Both the mother and her child were only able to be isolated in separate rooms. In the absence of exclusion of healthcare workers from the workplace and accurate respiratory isolation, vaccination could prevent the spread of measles in our setting.
It has been shown that nosocomial transmission of measles primarily affects children and infants, and young children acquiring measles nosocomially may have significantly higher complication rates, case-fatality rates, and longer recovery times compared to those acquiring the infection in the community [Reference Foulon13, Reference Biellik and Clements14]. Because the risk of exposure to measles for those children who are seriously ill may be higher, prompt PEP may be a life-saving measure. While limitations for vaccination is very rare in children, measles vaccine is the first choice for PEP [Reference Watson15]. The WHO recommends that the age for administration of measles vaccine should be lowered to 6 months during outbreaks where high attack rates are anticipated. However, effectiveness of PEP with MMR vaccine in children aged <1 year, especially in those aged <9 months, is not well established. We vaccinated 10 of the 44 exposed children, four of whom were aged between 6 and 9 months without any immune deficiency. Vaccination was effective in preventing the spread of measles to these children. For those infants vaccinated between ages 6 and 9 months, a second dose was planned to be administered as soon as possible after 9 months since the second dose at that time is important, as the serological response to vaccine given before age 9 months may be significantly lower, resulting in lower levels of protection.
Immunoglobulin administration may be preferable when vaccination is not possible and the risk of acquiring infection is higher. Nine children in our facility were given IVIG for PEP and none developed measles. Three of nine children who received IVIG (patient nos. 2, 4, 5 in Table 1) in this study can be seen as being eligible for vaccination because of being older than 6 months; however, we preferred to administer IVIG in view of their immune status because of their underlying condition and the concern of non-response. The child of the index case also received IVIG because of her underlying disease, unexplained fever and negative serology. It is uncertain whether the administration of IVIG masked the appearance of rash on her follow-up. Because of the negative serological results, despite her having received one dose of MMR vaccine, she was scheduled to receive a second dose of MMR vaccine. Vaccination schedule of the other children who had received IVIG and were eligible for vaccination was also planned to be completed. We had to administer IVIG to nine children because of their underlying condition and the lack of hyper-immune measles gamma globulin in Turkey. Intravenous immunoglobulin was 100% effective for preventing the spread of measles in our facility. Although passive immunoprophylaxis can be effective in certain situations for the prevention of hospital outbreaks, administration of serum immunoglobulin should not be used for outbreak control in the community.
Acting wisely before dealing with a case of measles in a facility is also important for prevention of sustained transmission. It was fortunate that the mother was diagnosed after an outbreak emerged in the community that raised suspicion in our hospital. At the time of the mother's diagnosis, an electronic database had been introduced to monitor the threat in the community, but it has not yet been fully implemented. There would have been more unvaccinated susceptible healthcare workers with the inevitable severe consequences if the database had not been introduced. Thirteen employees lacking documentary evidence of immunization were those who delayed the serological testing. These findings suggest the need for a comprehensive policy for vaccination of healthcare staff in our hospital. Proof of immunization or immunity should be required of all staff before or within the first few weeks of employment in order to prevent the introduction of measles into the hospital setting. Because elimination of the nosocomial transmission of measles will not be absolutely possible until the eradication of the disease, certain strategies applicable to all facilities to minimize nosocomial spread are needed. Every facility should maximize awareness in healthcare staff, and prepare its own infection control recommendations for measles based on its own resources.
CONCLUSION
Measles is highly preventable by adequate PEP with IVIG or vaccination in a healthcare setting that lacks the benefit of a negative pressure room.
DECLARATION OF INTEREST
None.




