INTRODUCTION
Bartonella henselae is the causative agent of cat-scratch disease (CSD) [Reference Chomel1, Reference Chomel2] and causes bacillary angiomatosis and bacillary peliosis hepatis in immunocompromised patients [Reference Welch3–Reference Florin, Zaoutis and Zaoutis5]. B. clarridgeiae has also been implicated as a possible aetiological agent of CSD in both immunocompromised and immunocompetent subjects [Reference Clarridge6, Reference Kordick7]. B. henselae can be transmitted to humans by a scratch or bite from an infected cat, while cat fleas (Ctenocephalides felis) and ticks (Ixodes rucinus) also play a role in transmission of the bacteria in cats [Reference Chomel8–Reference Billeter11].
Domestic and/or stray cats are reported to be a major reservoir of B. henselae and B. clarridgeiae in several countries [Reference Boulouis12, Reference Breitschwerdt13]. Recently, B. koehlerae was isolated from naturally infected cats and from a human patient with endocarditis [Reference Droz14, Reference Avidor15]. Therefore, cats serve as a significant reservoir for several zoonotic Bartonella spp. It has been reported that B. henselae is highly prevalent in domestic and/or stray cats in Asian countries, e.g. the Philippines [Reference Chomel16], Indonesia [Reference Marston17], Thailand [Reference Maruyama18], Taiwan [Reference Chang19], and Japan [Reference Maruyama20]. Several studies have shown that B. henselae can be classified into two types by 16S rRNA typing PCR: type I is predominant in cats in Asian countries [Reference Chomel16, Reference Maruyama18–Reference Maruyama20] while type II is predominantly isolated from cats in European countries [Reference Heller21–Reference Fabbi25].
B. vinsonii subsp. berkhoffii causes endocarditis, cardiac arrhythmias, and myocarditis in dogs, which are a reservoir of this organism, and has been isolated from a human patient with endocarditis [Reference Breitschwerdt26–Reference MacDonald28]. Furthermore, recent studies have suggested that other Bartonella spp. are able to infect dogs [Reference Gundi29]. In particular, isolation of B. clarridgeiae has been reported from a dog with aortic valve endocarditis [Reference Chomel30], and CSD osteomyelitis and lymphadenopathy can be caused by the scratch of B. henselae-infected dogs [Reference Keret31, Reference Tsukahara32]. In addition, B. washoensis has been isolated from a dog with endocarditis and a human patient with fever and myocarditis [Reference Chomel, Wey and Kasten33, Reference Kosoy34]. These data suggest that dogs, like cats, can be infected by several zoonotic Bartonella spp. and may serve as a source of infection of the bacteria.
The prevalence of Bartonella spp. in cats and dogs has not been thoroughly investigated in the Bangkok metropolitan areas, Thailand. Therefore, we investigated the prevalence of Bartonella infection in pet and stray cats and dogs in order to identify the predominant species and 16S rRNA type of Bartonella in those animals in the Bangkok metropolitan areas.
MATERIALS AND METHODS
Animal blood sampling
From June 2001 to February 2003, blood samples from 312 cats (288 strays, 24 pets) and 350 dogs (296 strays, 54 pets) were collected in the Bangkok metropolitan areas for this study. Stray animals were captured in Buddhist monasteries by monastery caretakers and our staff after receiving permission from the Buddhist monks. The samples of pet animals were collected at the Veterinary Teaching Hospital of Kasetsart University. Before sample collection, the general condition of animals was examined thoroughly, and the sex was noted. Stray cats were restrained by administration of an intramuscular injection of ketamine (10 mg/kg) and xylazine (1–2 mg/kg). Blood samples of the animals were aseptically collected from the jugular vein of cats and saphenous vein of dogs. Stray animals were immediately released after blood collection.
Blood samples (2 ml) from each animal were immediately placed into sterile EDTA tubes (Venoject II, Termo, Japan) and sent to the Laboratory of Veterinary Public Health, Nihon University, under frozen conditions with dry ice. The samples were kept at −80°C until examined.
Isolation and identification of Bartonella spp. from blood samples of cats and dogs
The frozen blood samples were thawed at room temperature and centrifuged at 3800 rpm for 70 min. After centrifugation, the supernatant was removed, 120 μl supplemented medium 199 (Gibco, USA) was added to the sediment, and the contents of the tube were mixed thoroughly [Reference Maruyama20]. A 100 μl sample of the mixture was plated on two heart infusion agar plates (Difco, USA) containing 5% defibrinated rabbit blood. The plates were incubated at 35°C under 5% CO2 for 4 weeks. The plates were checked for colony formation and fungal contamination weekly during the incubation period. After the 4 weeks incubation, 3–5 colonies with genus Bartonella morphology (small, round, grey, and rough colonies) were selected from each plate and subcultured using the same incubation conditions.
Genomic DNA was extracted from the isolates using the Instagene Matrix (Bio-Rad, USA). Species of the isolate was identified directly by PCR analysis of the 16S–23S rRNA inter-gene spacer region (ITS) [Reference Jensen35]. The PCR of ITS was performed with an iCycler (Bio-Rad) using a 20 μl mixture containing 20 ng extracted DNA, 200 μm each of dATP, dGTP, dCTP and dTTP, 1·5 mm MgCl2, 0·5 U Taq DNA polymerase (Promega, USA), and 1 pmol of each primer. The primer pair and the PCR conditions were followed as previously described [Reference Jensen35]. DNA from B. henselae ATCC 49882, B. clarridgeiae ATCC 51734, and B. vinsonii subsp. berkhoffii ATCC 51672 were used as positive PCR controls. Following PCR analysis, the amplicons were analysed by electrophoresis on 3% agarose gels. The samples were identified as B. henselae, B. clarridgeiae, or B. vinsonii subsp. berkhoffii if the amplified ITS DNA fragment had a size of 172, 154, or 260 bp, respectively [Reference Jensen35].
16S rRNA typing of B. henselae
B. henselae 16S rRNA typing was performed as previously reported [Reference Bergmans22] with a minor modification. Briefly, the primer pair of 16SF (5′-AGAGTTTGATCCTGGCTTCAG-3′) and BH1 (5′-CCGATAAATCTTTCTCCCTAA-3′) or BH2 (5′-CCGATAAATCTTTCTCCAAAT-3′) were used for the identification of B. henselae types I or II, respectively. The PCR was performed with an initial denaturation at 95°C for 3 min; 30 cycles of denaturation at 95°C for 20 s, annealing at 56°C for 30 s, and extension at 73°C for 1 min; and final extension at 73°C for 5 min. Following PCR, the amplicons were analysed by electrophoresis on 3% agarose gels.
DNA sequencing analysis
When an isolate was identified as B. clarridgeiae by species-specific PCR targeting for ITS region, the PCR amplicons were purified using a commercial kit (Quantum Prep Freeze 'N Squeeze DNA Gel Extraction Spin Columns; Bio-Rad), and subcloned using the pGEM-T Vector System (Promega) and E. coli strain DH5α (Nippon gene, Japan). Sequence analysis of subcloned DNA fragments was performed by the dye terminator method with T7 and SP6 primers, using an Applied Biosystems Model 310 Genetic Analyzer (Applied Biosystems, USA). The data were assembled and searched for sequence similarity with the database using GENETYX-win software, version 6 (Genetix Corp., Japan.) and the BLAST service in GenBank/EMBL/DDBJ.
Statistical analysis
The results were analysed by 2×2 tables and χ2 test was used to determine statistical significance. A P value <0·05 was considered to be significant.
RESULTS
Bartonella spp. were isolated from 47 (15·1%) of 312 cats examined (Table 1). The prevalence in stray cats was 16·3% (47/288), compared to no Bartonella being isolated from pet cats (0/24). Prevalence in males (21·6%, 25/116) was significantly higher than in females (11·9%, 21/176) (P<0·05).
Table 1. Prevalence of Bartonella infection in cats and dogs in the Bangkok metropolitan areas, Thailand
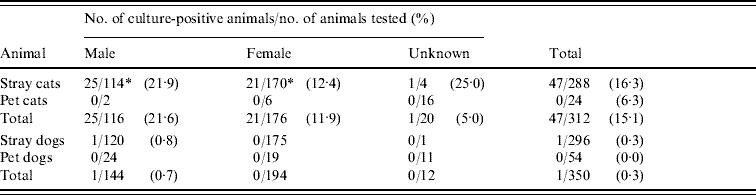
* P<0·05.
Of Bartonella bacteraemic cats, 45 (95·7%) of 47 cats were infected with only B. henselae. Of these 45 cats, 40 (88·9%) were infected only with B. henselae type I and four (8·9%) were infected only with B. henselae type II. Only one cat (2·2%) was infected with both types. Of the remaining two cats, one was only infected with B. clarridgeiae and the other was infected by B. henselae type I and B. clarridgeiae (Table 2).
Table 2. Distribution of Bartonella spp. or Bartonella henselae types in cats and dogs in the Bangkok metropolitan areas, Thailand
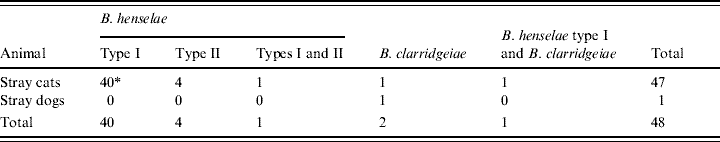
* Number of animals from which the B. henselae types were isolated.
In this study, one (0·3%) out of 296 stray dogs was culture-positive for Bartonella, although the level of bacteraemia was low at 82 c.f.u./ml. This dog had no obvious clinical symptoms at sampling. No Bartonella was detected in the 54 pet dogs studied (Table 1). Species-specific PCR identified the dog isolates as B. clarridgeiae (Table 2). The isolates were confirmed to be B. clarridgeiae by DNA sequencing with 100% similarity of the 114 bp ITS region.
DISCUSSION
There have been several reports of Bartonella infection in cats in Asian countries, with the bacteraemia prevalence varying by country: 64·3% (9/14) in Indonesia [Reference Marston17], 61·3% (19/31) in the Philippines [Reference Chomel16], 27·6% (76/275) in Thailand [Reference Maruyama18], 19·1% (25/131) in Taiwan [Reference Chang19], and 7·2% (50/690) in Japan [Reference Maruyama20]. In the present study, the Bartonella bacteraemia prevalence in cats was found to be 15·1% (47/312) in the Bangkok metropolitan areas. Collectively, these data indicate that Bartonella is widely distributed throughout Asia, with a higher prevalence especially in southern Asian countries than in northern temperate countries. In agreement with this observation, it was suggested that cats in a warm humid environment had a higher seroprevalence of B. henselae than those in a cold dry environment [Reference Jameson36].
Of the 47 Bartonella-positive stray cats, 45 (95·7%) were only infected with B. henselae, one was only infected with B. clarridgeiae, and the other was infected with both B. henselae and B. clarridgeiae. These data suggest that B. henselae is the predominant Bartonella spp. in cats in Thailand. Of the 45 stray cats with only B. henselae, 16S rRNA typing showed that 40 (88·9%) had type I, four (8·9%) had type II, and one (2·2%) had both types I and II (Table 2). One stray cat was infected with both B. henselae type I and B. clarridgeiae. Thus, B. henselae type I was the predominant type in stray cats in the Bangkok metropolitan areas. B. henselae type I is reported to be the predominant type in cats in other Asian countries, e.g. the Philippines (100%, 17/17) [Reference Chomel16], Taiwan (76·0%, 19/25) [Reference Chang19], and Japan (97·8%, 44/45) [Reference Maruyama20]. On the other hand, type II is the prevalent type in European countries, such as Denmark (95·2%, 20/21) [Reference Chomel24], Germany (94·7%, 18/19) [Reference Arvand23], The Netherlands (66·7%, 14/21) [Reference Bergmans22], Italy (61·1%, 80/131) [Reference Fabbi25], and France (51·4%, 18/35) [Reference Heller21]. These results indicate that the distribution of B. henselae 16S rRNA types I and II is considerably different between Asian and European countries.
It has been reported that there was no significant difference in the prevalence of Bartonella between male and female cats in Thailand [Reference Maruyama18]. However, in the present study, the prevalence in male cats (21·6%, 25/116) was significantly higher than in female cats (11·9%, 21/176) in the Bangkok metropolitan areas (P<0·05). It may be speculated that the differences between these results is due to male cats having more opportunities to be scratched or bitten by other cats while protecting their territories in the limited Bangkok metropolitan areas compared to other rural areas in Thailand.
In this study, B. clarridgeiae was isolated from only one out of 350 dog samples (0·3%). This is the first report of B. clarridgeiae isolation from a dog in Thailand, although the number of bacteria in the blood was low at 82 c.f.u./ml. Although B. clarridgeiae has been mainly isolated from cats, it has been isolated from the blood of a dog with aortic valve endocarditis in the USA [Reference Chomel30] and from the blood of dogs in Gabon [Reference Gundi29]. In the present study, the dog infected with B. clarridgeiae appeared healthy on physical examination. In addition, the prevalence of B. clarridgeiae bacteraemia in dogs was very low suggesting that dogs are more likely to be accidental hosts of B. clarridgeiae in Thailand.
In a previous report, 38% of sick dogs in Thailand, with fever, anaemia, or thrombocytopenia, tested seropositive for B. vinsonii subsp. berkhoffii [Reference Suksawat37]. Although B. vinsonii subsp. berkhoffii seroprevalence in dogs was not examined in this study, no dogs were found to harbour the species in those blood samples. Therefore, the prevalence of B. vinsonii subsp. berkhoffii in dogs should be relatively low in the Bangkok metropolitan areas.
Further studies are required to clarify the detailed prevalence and distribution of Bartonella spp. in cats and dogs in Asian countries, and to provide data for the prevention of bartonellosis from these animals.
ACKNOWLEDGEMENTS
This work was supported by a Grant for Academic Frontier Project ‘Surveillance and Control for Zoonoses’ from the Ministry of Education, Culture, Sports, Science, and Technology and Health Science Grants for research on Emerging and Re-emerging Infectious Diseases from the Ministry of Health, Labor, and Welfare, Japan.
DECLARATION OF INTEREST
None.




