INTRODUCTION
Salmonella is an enteric bacterial pathogen that causes foodborne illness in humans. In Japan, more than 10 000 cases of foodborne salmonellosis were reported annually between 1996 and 1999 [1]. During this period, Salmonella enterica subsp. enterica serovar Enteritidis (hereafter S. Enteritidis) was the most frequently isolated serovar. S. Enteritidis infections are frequently associated with the consumption of eggs, therefore the Enforcement Regulations of the Food Sanitation Law were partially amended in 1998 to ensure the safe distribution of raw shell eggs and liquid egg products. Although the number of foodborne salmonellosis cases has significantly decreased since 2000, Salmonella remains one of the two main causative agents of bacterial food poisoning [2].
The percentage of S. Enteritidis has decreased in Salmonella isolates from human salmonellosis cases, whereas the percentage of S. Infantis has increased [2]. In Japan, S. Infantis is frequently isolated from broilers [Reference Murakami3–Reference Ishihara7], but rarely isolated from beef and dairy cattle and swine [Reference Asai4, Reference Kishima8, Reference Ahmed9]. According to Asai et al. [Reference Asai10], 94·0% of S. Infantis isolates from Japanese broilers and chicken meat products between 2001 and 2003 were resistant to both oxytetracycline (OTC) and dihydrostreptomycin (DSM). Hamada et al. [Reference Hamada11] reported that S. Infantis isolates from humans between 1994 and 2003 were resistant to both streptomycin and tetracycline. We recently reported that S. Infantis was isolated from 14 (4·1%) of 338 laying-hen farms in Japan between 2007 and 2008, and 13/14 S. Infantis isolates were susceptible to both DSM and OTC [Reference Sasaki12]. Noda et al. [Reference Noda13] reported that the geno-types of S. Infantis isolated from humans were similar to those isolated from chicken meat, whereas no common genotypes were found in human and chicken egg isolates. The results of these studies suggest that human S. Infantis infection cases in Japan originate from the consumption and handling of broiler meat.
Human salmonellosis is generally self-limiting, so antimicrobial therapy is not usually required. However, invasive infections require treatment with antimicrobial agents. It is important to monitor the rate of antimicrobial resistance in Salmonella isolated from broiler farms.
The purpose of this study was to determine the prevalence and antimicrobial susceptibility of Salmonella isolated from broiler flocks in Japan.
MATERIALS AND METHODS
Broiler flocks
Fourteen broiler production companies that account for more than 50% of domestic Japanese chicken production participated in this study. Samples were collected from 288 flocks engaged in conventional production between November 2007 and February 2010. A total of 265 broiler farms investigated were located in seven regions of Japan, i.e. Tohoku, Kanto, Tokai, Kinki, Chugoku, Shikoku and Kyushu (Fig. 1). A flock was defined as a group of birds raised in a broiler house during the same period of time.
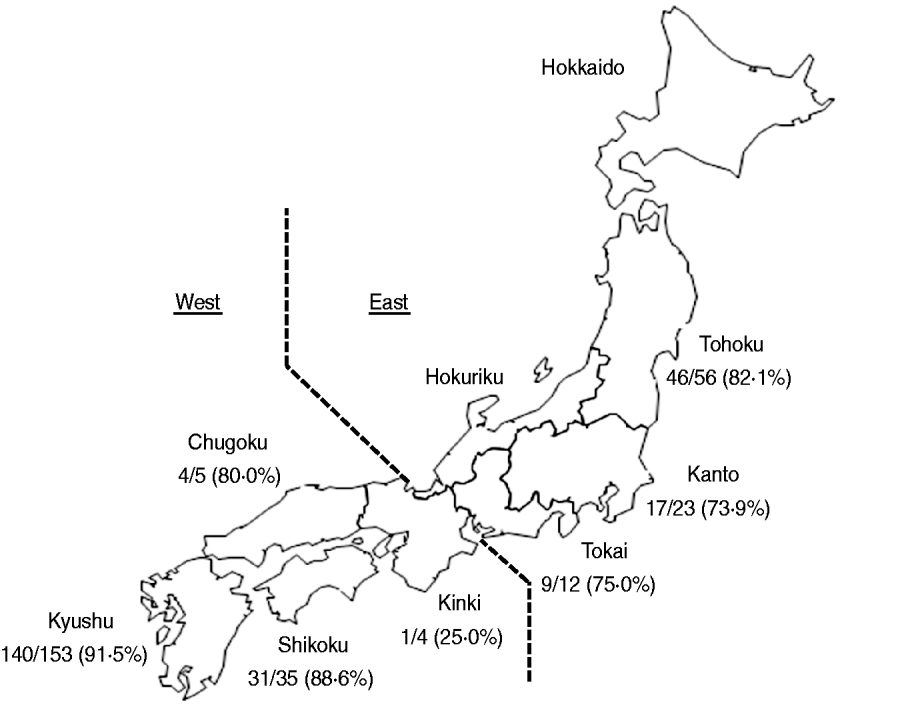
Fig. 1. Map of the study regions. Samples were obtained from seven regions (Tohoku, Kanto, Tokai, Kinki, Chugoku, Shikoku, Kyushu). Below each region label is the number of Salmonella-positive flocks and the total number of flocks tested.
Samples
Sampling of a selected flock was conducted on a farm only once during the final 2 weeks of the production cycle. In 23 farms, sampling was also conducted at the end of the next production cycle. Five pooled fresh caecal dropping samples were taken from a house in the respective farms. Each pooled fresh caecal dropping sample (~10 g) consisted of caecal droppings from several birds. Samples were placed in plastic vials and transported under refrigeration to the Japan Food Research Laboratories (JFRL) or the Research Institute for Animal Science in Biochemistry and Toxicology (RIAS). The samples were kept refrigerated in the laboratories until examination, which was performed within 48 h of their arrival.
Bacteriological examination
Ten grams of pooled fresh caecal droppings were mixed with 90 ml buffered peptone water (Eiken Chemical, Japan) and maintained at room temperature before being cultured at 35°C for 22±2 h. Subsequently, 0·1- and 1-ml aliquots of the culture were added to 10 ml Rappaport-Vassiliadis broth (bioMérieux Japan Ltd, Japan) and 10 ml tetrathionate broth (Merck Ltd, Japan), respectively. After incubation at 42°C for 22±2 h, each culture was streaked onto two selective isolation agar plates, xylose-lysine-deoxycholate agar (bioMérieux) and Brilliant Green agar (bioMérieux). Candidate colonies were biochemically identified. Salmonella isolates were tested by slide agglutination with O antisera (Denka Seiken Co., Japan). One of each different O serogroup isolates was stored in tryptone soya broth containing 10% glycerol at −80°C. All Salmonella isolates were sent to RIAS or the Machida Hygiene Control Laboratory (MHCL) for tube agglutination tests using H antisera (Denka Seiken). Serovars were determined on the basis of reaction with O and H group antigens according to the Kauffmann–White method [Reference Poppoff14].
Antimicrobial susceptibility testing
The minimum inhibitory concentration (MIC) was determined using the agar dilution method of the Clinical and Laboratory Standards Institute (CLSI) [15]. Escherichia coli ATCC 25922 was used as the quality control strain. The resistance breakpoints defined by CLSI were used [16]. Breakpoints not defined by CLSI were obtained from a previous report [Reference Esaki17]. Antimicrobial susceptibility tests were conducted at RIAS or MHCL.
RESULTS
Salmonella prevalence and serovars
Salmonella was obtained from 248 (86·1%, 95% confidence interval 82·1–90·1) of 288 broiler flocks. The prevalence of Salmonella in broiler flocks in each of the investigated regions was greater than 73%, with the exception of Kinki region (Fig. 1). The prevalence (91·5%, 140/153) of Salmonella in Kyushu region was statistically (P=0·004, Fisher's exact test) higher than that (80·0%, 108/135) in the other regions.
In all, 285 isolates were obtained from 248 flocks. They were serotyped into 17 serovars and 12 untypable Salmonella (Table 1). The three main serovars were S. Infantis, S. Manhattan and S. Schwarzengrund. S. Infantis was the most frequent serovar, and it was isolated from 176 (61·1%) flocks in all seven regions where the study was conducted. S. Manhattan and S. Schwarzengrund were frequently found only in the western part of Japan.
Table 1. Frequency distribution of Salmonella serovars in broiler flocks
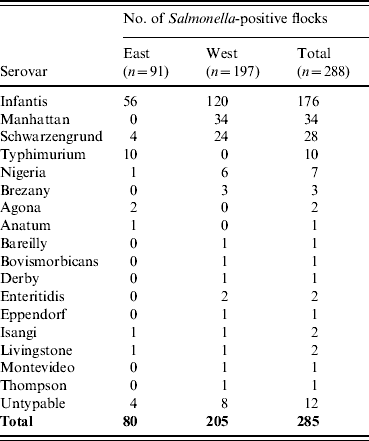
n, Number of flocks tested.
East (Tohoku, Kanto, Tokai); West (Kinki, Chugoku, Shikoku, Kyushu).
Sampling was conducted on a second occasion for 23 farms at the end of the next production cycle. Twenty of the 23 farms were Salmonella-positive both before and after the production gaps, while the remaining three farms were negative on both occasions. Moreover, Salmonella isolates obtained before and after the production gaps were identical in terms of serovars and antimicrobial resistance profiles on 14 (70·0%) of the 20 Salmonella-positive farms (data not shown).
Antimicrobial susceptibility
High antimicrobial resistance rates were observed against OTC (90·2%), DSM (86·7%) and ampicillin (ABPC) (36·5%) (Table 2). All of the isolates in this study were susceptible to gentamycin (MIC ⩽1), apramycin (MIC ⩽8), enrofloxacin (MIC ⩽1) and fosfomycin (MIC ⩽32). Sulphadimethoxine showed no activity against all isolates (MIC ⩾128). Of the 285 isolates, 258 (90·5%) were resistant to two or more of the antimicrobials tested in this study (Table 3). In particular, 243 (85·3%) isolates were resistant to both DSM and OTC. For S. Infantis, 160 (90·9%) of 176 isolates were resistant to both DSM and OTC. With regard to the β-lactam antimicrobial agents [ABPC, cefazolin (CEZ) and ceftiofur (CFT)], 81 (46·0%), 71 (40·3%) and 67 (38·1%) of 176 S. Infantis isolates were resistant to ABPC, CEZ and CFT, respectively.
Table 2. Antimicrobial susceptibility of Salmonellaisolates from broiler flocks
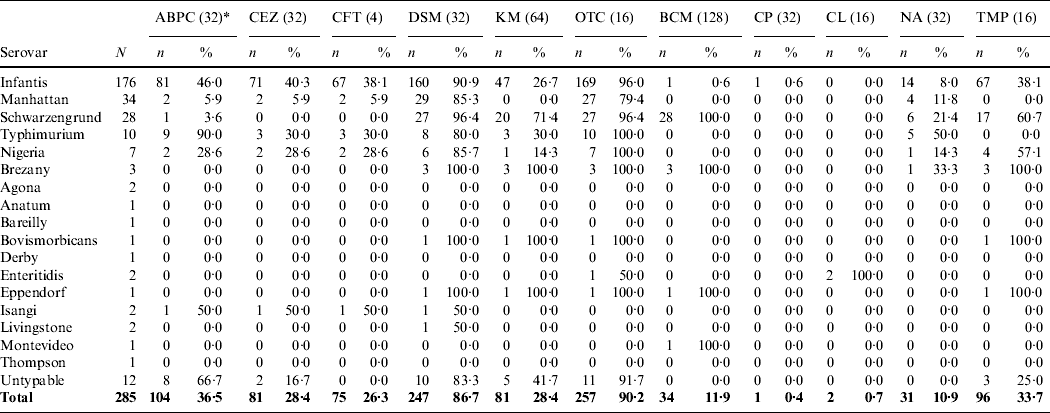
* Values in parentheses are the minimum inhibitory concentration (mg/l) of the breakpoint.
ABPC, Ampicillin; CEZ, cefazolin; CFT, ceftiofur; DSM, dihydrostreptomycin; KM, kanamycin; OTC, oxytetracycline; BCM, bicozamycin; CP, chloramphenicol; CL, colistin; NA, nalidixic acid; TMP, trimethoprim.
Table 3. Antimicrobial resistance profiles of Salmonella isolates (n=285)

ABPC, Ampicillin; BCM, bicozamycin; CEZ, cefazolin; CFT, ceftiofur; CL, colistin; CP, chloramphenicol; DSM, dihydrostreptomycin; KM, kanamycin; NA, nalidixic acid; OTC, oxytetracycline; TMP, trimethoprim.
DISCUSSION
Two previous reports of Salmonella prevalence in broiler flocks in Japan were based on samples taken in slaughterhouses [Reference Shahada6, Reference Limawongpranee18]. Surveillance in Tochigi Prefecture in Kanto region between 1995 and 1997 and in Kagoshima Prefecture in Kyushu region between 1998 and 2003 demonstrated a high prevalence of 64·3% (18/28) and 70·6% (178/252), respectively [Reference Shahada6, Reference Limawongpranee18]. Although their results are not comparable to our results due to differences in sampling and isolation methods, Salmonella prevalence in broiler flocks could remain high throughout the country. In the present study, S. Infantis was the most frequent serovar, isolated from 176 (61·1%) flocks. The prevalence of Salmonella was statistically higher in Kyushu region (91·5%), where 45% of Japanese broiler meat production takes place [19], than that in the other regions (80·0%). The most frequently isolated serovar in this region was also found to be S. Infantis. Therefore, efforts to reduce human salmonellosis caused by S. Infantis should focus on the establishment of control measures in broiler farms, especially, those located in Kyushu region. Salmonella isolates obtained before and after the production gaps were identical in terms of serovars and antimicrobial resistance profiles on 14 (70·0%) of the 20 Salmonella-positive farms. We recently showed that most broiler farms have adopted appropriate biosecurity measures, such as disinfection of vehicles and equipment before entering the farm, the practice of ‘all-in/all-out’ management at the farm level, changing of working clothes every day, and disinfection of footwear at each house entrance [Reference Sasaki20]. These results strongly suggest that the currently adopted biosecurity measures are not sufficiently effective or they have not been implemented properly, thereby allowing the persistence of Salmonella on broiler farms. Alternatively, Salmonella might be brought to broiler houses after production gaps from reservoirs located in the surrounding environment. Further studies are necessary to identify the mechanism of the persistence or re-introduction of Salmonella to broiler farms in Japan, and adequate countermeasures need to be established to address these problems.
Since the late 1990s, S. Infantis has been the main serovar isolated from broilers in Japan [Reference Murakami3–Reference Ishihara7]. S. Infantis seems to have predominated in broiler flocks in Japan during this decade. Such predominance by S. Infantis has not been commonly observed in other countries [21, Reference Mellor22], the reasons for which cannot be explained from currently available information and require further investigation. S. Manhattan was the second most common serovar, but it was seldom isolated from broiler flocks before the present study. The National Veterinary Assay Laboratory (NVAL), which has managed the Japanese Veterinary Antimicrobial Resistance Monitoring (JVARM) System since 1999, reported that S. Manhattan was first obtained from the faecal samples of broilers in 2007 [23]. In the present study, S. Manhattan was isolated from broiler flocks located in the western part of Japan during the investigation period. The serovar may have settled in the western part of Japan. Since 2005, S. Schwarzengrund was previously detected only in the Kinki, Chugoku and Kyushu regions, which are located in the western part of Japan [23, Reference Asai24]. S. Schwarzengrund isolates had never been detected in the eastern part of Japan before 2009, but four isolates were detected in the Tokai region in 2009 during this study, which is the westernmost region of the eastern part of Japan. These results suggest that this serovar has spread from the western parts to the eastern parts of Japan.
With the exception of the β-lactam antimicrobial agents (ABPC, CEZ, CFT) the patterns of antimicrobial resistance were very similar to those reported in previous studies [Reference Asai4, Reference Ishihara7]. Resistance rates against β-lactam antimicrobial agents were higher than those reported in previous studies. In particular, the resistance rates against CEZ (a first-generation cephalosporin) and CFT (a third-generation cephalo-sporin) were notable because cephalosporins have not been approved for poultry disease treatment in Japan, under the Japanese Pharmaceutical Affairs Law. Ishihara et al. [Reference Ishihara7] reported that two (1·8%) of 111 isolates from broilers were resistant to CFT in 1999. According to JVARM, two (4·3%) of 47 Salmonella were resistant to CEZ in isolates from broilers during 2006, but no Salmonella resistant to CEZ were isolated between 2000 and 2005 [23]. Shahada et al. [Reference Shahada25] recently reported that S. Infantis resistance to cephalosporins has emerged in broilers from 2005 based on a survey conducted at a slaughterhouse between 2004 and 2006, where 11 (9·1%) of 120 S. Infantis isolates were found to be resistant to cefotaxime (a third-generation cephalosporin). However, none of the 135 S. Infantis isolates sampled from broilers at a slaughterhouse between 1998 and 2003 were found to be resistant [Reference Shahada6]. In this study, 71 (40·3%) and 67 (38·1%) of 176 S. Infantis isolates were resistant to CEZ and CFT, respectively. These results suggest that cephalosporin resistance rates have increased in Salmonella isolated from broilers since 2006. S. Infantis resistant to cephalosporins might be already causing human salmonellosis via broiler meat consumption although there is no way to gauge the real impact on human health due to lack of studies on antimicrobial susceptibility of S. Infantis in human salmonellosis cases. Therefore, the impact of a growing resistance to cephalosporins in broilers cannot be dismissed. Further studies are needed to clarify the reasons why Salmonella resistance to cephalosporins is prevalent in broiler flocks in Japan.
ACKNOWLEDGMENTS
We express our gratitude to the broiler production companies who participated in this study, and JFRL, RIAS and MHCL for their cooperation. This study was funded by the Ministry of Agriculture, Forestry and Fisheries of Japan.
DECLARATION OF INTEREST
None.






