INTRODUCTION
Campylobacter spp. are the most common bacterial causes of gastroenteritis in developed countries. In Finland, around 3500–4000 Campylobacter cases have been reported in recent years (http://www3.ktl.fi). Most of the cases are sporadic, which complicates the identification of infection sources. The incidence of Campylobacter infections is highest in the summer months showing a peak in July [Reference Nylen1, Reference Iivonen2]. Finnish clinical microbiology laboratories are obliged to report their Campylobacter findings to the National Infectious Disease Register (NIDR) maintained at the Department of Infectious Disease Surveillance and Control, National Institute for Health and Welfare (THL) (formerly National Public Health Institute, KTL). Patients were asked about their travel history since 2004. In that year, information was obtained from 61% of cases. Of those, 68% had been abroad just prior to becoming ill [Reference Iivonen2]. However, the actual proportions of domestic and imported infections have not been reliably determined because of the high percentage of data without known travel history.
In this study, Campylobacter strains isolated from patients were collected for epidemiological typing. Patients were asked about their travel histories to obtain reliable information regarding the foreign or domestic origin of the Campylobacter infections. The overall incidence, and demographical and seasonal distribution of the infections in Finland were also investigated. In addition, serotypes of domestic C. jejuni strains were analysed to find features characteristic of the Finnish Campylobacter strains. This knowledge will help in directing epidemiological investigations and focusing on control measures for Campylobacter infections.
METHODS
Reporting findings
Between 1 January 2002 and 31 December 2005, 14 361 Campylobacter infections were reported to NIDR. The NIDR provided data on the overall incidence, seasonal changes in the number of Campylobacter infections and demographical distribution of the cases diagnosed in Finland.
Strains studied
Campylobacter strains (n=2364, 16% of cases reported to NIDR in 2002–2005) were collected during a 3-year period (from 1 July 2002 to 31 June 2005) and represented all Campylobacter strains found in nine Finnish clinical microbiology laboratories of nine hospital districts. The laboratories enquired about the patient's travel history, and this together with the date when the specimen was taken were recorded on a special form accompanying the patient's strain submitted to Enteric Bacteria Laboratory (EBL) of KTL (presently the Bacteriology Unit of THL) for further testing. The strain was regarded as being associated with foreign travel if the patient had travelled abroad and the onset of symptoms was within 10 days or the specimen was taken within 17 days after the patient's return. Of the 2364 strains collected, 1407 C. jejuni strains (60%) were further analysed by heat-stable serotyping. These included all C. jejuni isolates from domestic (n=622) and foreign travel-related (n=785) infections isolated during a 2-year period (July 2002–June 2003 and July 2004–June 2005). Only one strain per case was studied.
Identification and serotyping
Preliminary species identification was carried out at the clinical laboratories by hippurate hydrolysis test. Hippurate-positive strains were identified as C. jejuni. The species identification of hippurate-negative strains was carried out in EBL by PCR as described previously [Reference Nakari, Puhakka and Siitonen3]. The strains were subcultured twice on blood agar and grown for 48 h at 42°C in a microaerobic atmosphere. Serotyping was performed according to the Penner serotyping scheme [Reference Penner and Hennessy4] using a commercially available serotyping kit (Denka-Seiken Co. Ltd, Japan), which contains 25 absorbed antisera against heat-stable serotypes (1,44), 2, 3, (4,13,16,43,50), 5, (6,7), 8, 10, 11, 12, 15, 18, 19, 21, (23,36,52), 27, 31, 32, 37, 38, 41, 45, 52, 55, and 57. Non-typable strains were designated NT.
Statistical methods
Yates-corrected χ2 and Fisher's exact one-tailed tests (Epi-Info™ 3.3, Centers for Disease Control and Prevention, USA) were used to compare the proportions of serotypes in domestic infections with those in travel-associated infections. A P value of <0·05 indicated statistical significance.
RESULTS
Seasonal variation and incidence of all reported infections
In total, 14 361 Campylobacter cases were reported during 2002–2005. The seasonal variation in the number of cases was similar each year: low in winter, and peaking in July and August (Fig. 1). The majority of the 2364 cases for which the travel history was known, were of domestic origin during the seasonal peak, only 28–43% were associated with foreign travel, compared to 65–93% in winter (December–February, Fig. 1). The overall annual incidence varied from 61/100 000 in 2003 to 76/100 000 in 2005. The mean incidence over the 4-year period was highest, 148/100 000, in the 25–29 years age group (Fig. 2). The lowest incidences were detected in children aged 5–9 (21/100 000), and 10–14 (23/100 000) years, and in patients aged ⩾75 years (24/100 000). In children aged <5 years, the incidence was higher (33/100 000) than in older children. The most marked change in the incidence was the increase from 49/100 000 in the 15–19 years age group to 112/100 000 in the 20–24 years age group. From 0 to 14 years, the number of domestically acquired infections decreased, whereas the number of foreign travel-related infections increased (Fig. 2). The number of domestic infections remained constant in patients aged 20–59 years whereas there was more variation in the number of travel-associated infections. The proportion of foreign travel-related infections varied from 54% to 68% in patients aged 10–59 years, and from 13% to 45% in other patient groups (Fig. 2).
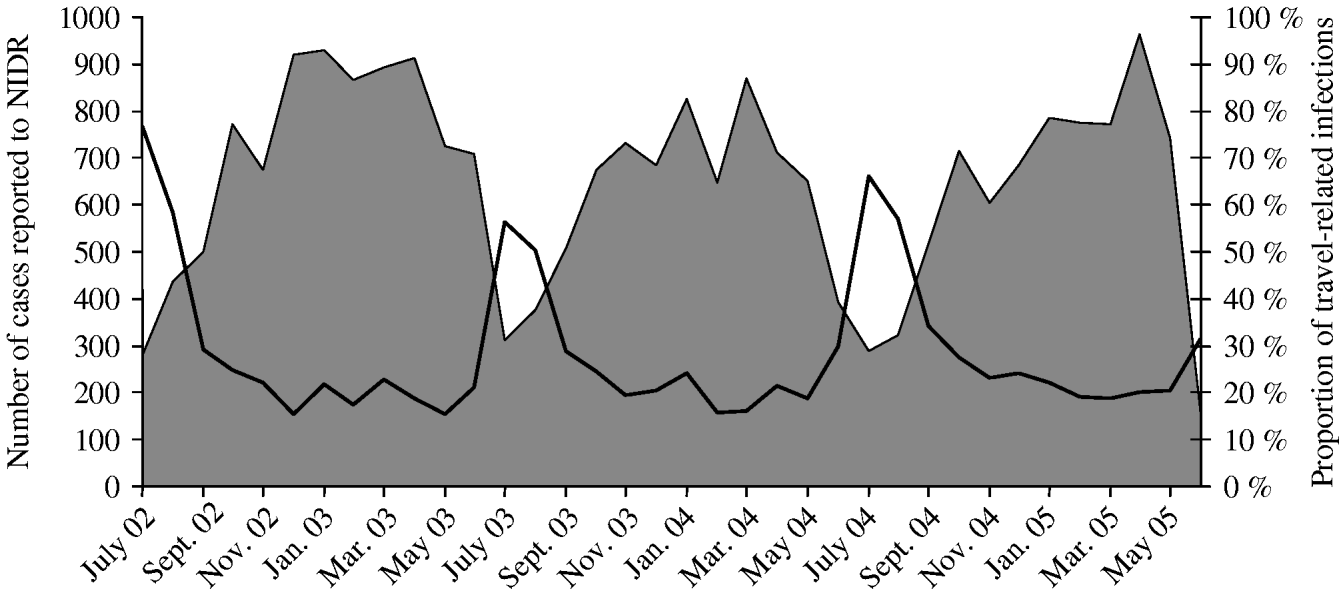
Fig. 1. Monthly incidence of Campylobacter cases reported to the National Infectious Disease Register (NIDR) between July 2002 and June 2005 (–––), and the proportion of foreign travel-related cases of the strains collected in this study (![]() , n=2364) during the same time period.
, n=2364) during the same time period.

Fig. 2. Mean incidence of Campylobacter infection in different age groups of patients in Finland between 2002 and 2005 as reported to the National Infectious Disease Register (n=14 361), and travel history of the patients in nine hospital districts between July 2002 and June 2005 (n=2364). ![]() , Domestic; □, travel related;
, Domestic; □, travel related; ![]() , travel history unknown.
, travel history unknown.
Data on species and serotype distributions
Of the 2364 strains collected, C. jejuni accounted for 92% (2184 strains), and C. coli for 7% (168). In addition, there were C. upsaliensis (n=8), C. fetus (n=2) and C. lari (n=2) strains. The data on the patients' travel history was available for 92% (2186) of the strains. Of the C. jejuni strains, 41% were of domestic origin, 51% were associated with foreign travel and data was unavailable for 8%. Of the 168 C. coli strains, 81% were associated with foreign travel, 17% were of domestic origin and data was unavailable for 2%.
The 1407 C. jejuni strains that were serotyped, represented all of the 21 specific serotypes and four serogroups included in the serotyping kit. In addition, 15 strains reacted with several antisera (mixed serotypes) and 593 strains (42%) were NT.
The most common serotypes were Pen 2 (14%), Pen 4-complex (8%), Pen 12 (6%) and Pen 1,44 (6%) (Table 1). Serotypes Pen 12, Pen 6,7 and Pen 27 were more common (12%, 6%, 3%, respectively) in domestic strains than in travel-associated strains (2%, 2%, 1%, P<0·001 for each). In contrast, majority of the isolates within serotypes Pen 1,44 (4% vs. 7%, P<0·05), Pen 3 (<1% vs. 6%, P<0·001) and Pen 37 (<1% vs. 3%; P<0·001) were from patients with a history of foreign travel (Table 1).
Table 1. Serotypes of the strains (n=1407) isolated from domestic (n=622) and foreign travel-associated (n=785) C. jejuni infections, and comparison of proportional data by χ2 test

n.s., Not significant; n.d., not determined.
Of the domestic C. jejuni strains, 71% were found in summer (June–August, n=442), 19% in autumn (September–November, n=121), 6% in winter (December–February, n=38), and 3% in spring (March–May, n=21). The distributions of the specific serotypes in the domestic strains followed the same seasonal trend showing a peak in summer and low incidences in other seasons, except for Pen 2. A higher proportion of Pen 2 strains was isolated in winter (18%) compared to the other serotypes (0–10%). Of the travel-associated strains, 33% (259 strains) were isolated in summer, 25% (199) in autumn, 22% (171) in winter and 20% (156) in spring. Pen 1,44 and Pen 6,7 showed a peak in summer (44% and 62%, respectively), and the majority of the Pen 12 strains were isolated in summer (44%) and autumn (50%). The other serotypes in travel-associated strains were evenly distributed seasonally.
To study the demographical distribution of the serotypes, patients were divided into four age groups: 0–19 (83/88 isolates from domestic/imported infections), 20–39 (176/348), 40–59 (212/282) and ⩾60 (151/62) years. In domestic infections, the strains of serotype Pen 12 were frequent (11–13%) in all age groups. Further, Pen 4-complex, Pen 6,7 and Pen 27 were evenly distributed (8–10%, 4–9% and 1–4%, respectively). Pen 2 was the most common serotype in patients aged 0–59 years (13–18%) but accounted for only 7% of the isolates from older patients. Pen 1,44 was more common (7%) in the 0–19 years age group than in older patients (3–4%). Of the travel-related strains, the proportions of Pen 2 and Pen 1,44 were lower (6% and 3%, respectively) in patients ⩾60 years than in younger patients (13–16% and 6–7%), whereas the proportion of Pen 4-complex was higher (16%) than in younger age groups (6–9%).
The nine clinical microbiology laboratories were grouped in three geographical regions, Southern (179/326 isolates from domestic/imported infections), Eastern (141/97) and Western (302/362) Finland, each with three laboratories to study the geographical distribution of the serotypes. In Southern Finland, the most common serotypes in domestic infections were Pen 12 (15%), Pen 2 (9%), Pen 1,44 (7%), and Pen 4-complex (6%); in Eastern Finland Pen 12 (12%), Pen 4-complex (12%), and Pen 2, Pen 6,7 and Pen 27 (6% of each) and in Western Finland Pen 2 (18%), Pen 12 (10%), Pen 4-complex (9%), and Pen 6,7 (8%). The lower frequency of Pen 2 in patients aged ⩾60 years was observed in Southern and Western Finland (5% and 9%, respectively) compared to younger patients (8–13% and 20–23%). In Eastern Finland, the frequency of Pen 2 was 19% in the 0–19 years age group, and 4–6% in older patients. Of the travel-associated infections, there were only minor geographical differences in the distributions of the serotypes.
DISCUSSION
We derived data on 14 361 human cases from NIDR, and serotyped results from a collection of 1407 C. jejuni strains isolated from patients with a known travel history. The aim was to investigate temporal, geographical and demographical trends of Campylobacter infections diagnosed in Finland in 2002–2005. In particular, we were interested in domestic cases, which were defined as not travelling abroad within 10 days prior to the onset of symptoms or 17 days prior to providing the specimen. The mean incubation period of Campylobacter infection is usually 2–5 days, with a range of 1–10 days [5, Reference Skirrow, Blaser, Nachamkin and Blaser6].
The mean incidence of Campylobacter infections was low in children (aged 0–14 years), but increased rapidly in the 15–29 years age group, and then decreased in older age groups. A peak in the incidence in young adults has been observed in many countries [Reference Friedman, Nachamkin and Blaser7] and it has been proposed that it is related to increased foreign travel in this age group [Reference Kapperud and Aasen8]. In our study, foreign travel-related and domestically acquired infections were analysed separately. In children aged <5 years the incidence was higher than in older children although the proportion of travel-associated infections was lower. Previous studies have also shown a higher incidence in children aged <5 years in many European countries, including Finland, USA and New Zealand [Reference Friedman, Nachamkin and Blaser7, Reference Koehler9, Reference Vierikko10]. This may be due to oversampling in this age group [Reference Kapperud and Aasen8] or potential risk factors in young children [Reference Koehler9, Reference Tenkate and Stafford11–Reference Schönberg-Norio13]. The number of both domestically acquired and travel-related cases almost doubled from the 15–19 years age group to the 20–24 years age group. Since the proportions of domestic and travel-related infections remained the same in these two age groups, increased foreign travel could only partly explain the increase in incidence. However, a higher rate of domestic infections in young adults in Finland was also reported in 1999 [Reference Vierikko10].
The annual number of domestic and travel-associated cases was about the same but the seasonal distribution was very different. The number of travel-associated cases was relatively constant throughout the year whereas domestic cases were infrequent in winter and increased considerably in summer. Our results showed that the seasonal peak observed in July and August in Campylobacter cases in Finland was mostly caused by domestic infections. Seasonal variations in human behaviour that may expose people to campylobacters, such as barbecue-prepared meals and attending outdoor parties, are similar to the seasonal distribution of Campylobacter infections [Reference Sopwith14, Reference Eberhart-Phillips15]. Moreover, the prevalence of C. jejuni in Finnish broiler flocks and cattle peak in July and August [Reference Perko-Mäkelä16, Reference Hakkinen, Heiska and Hänninen17]. The overall seasonal pattern of human cases and contamination of broiler flocks is similar in other Scandinavian countries as well [Reference Nylen1, Reference Hansson18–Reference Hofshagen and Kruse20].
The most common serotypes in domestic C. jejuni strains were Pen 2, Pen 12, Pen 4-complex, Pen 6,7, Pen 1,44 and Pen 27, each with a proportion of ⩾3%. In a previous study, the same serotypes were found to predominate [Reference Vierikko10]. The seasonal distribution of the infections caused by these serotypes followed the overall seasonal pattern of domestic Campylobacter infections in Finland. Of these serotypes, Pen 2, Pen 4-complex and Pen 1,44 were among the five most common types of travel-related strains, whereas Pen 12, Pen 6,7 and Pen 27 were significantly associated with domestic infections. The overall proportions of the two most common serotypes Pen 2 and Pen 12 in all domestic strains was about the same but their distribution differed. Pen 12, Pen 4-complex and Pen 6,7 were evenly distributed both geographically and across the age groups of the patients. Pen 2 was less common in patients aged ⩾60 years. A lower mean age of Finnish patients infected with a strain of serotype Pen 2 has been reported previously [Reference Schönberg-Norio13]. It has been suggested that people may develop immunity to the most common serotypes of C. jejuni, which leads to a reduced frequency of common serotypes in older age groups [Reference Miller21, Reference Linneberg22]. Another interesting feature was that the frequency of Pen 2 varied geographically between 18% in Western Finland and 6% in Eastern Finland. Moreover, Pen 2 strains were more prevalent than other serotypes in winter. For comparison, the seasonal, demographical and geographical distributions of the serotypes of strains isolated from travel-associated infections were also analysed. Of travel-associated strains, Pen 2 was evenly distributed seasonally whereas Pen 12, Pen 1,44 and Pen 6,7 peaked in summer and/or autumn. However, the differences found in the travel-associated strains may be of limited value because the destination countries varied both seasonally and depending on the age of the traveller.
The serotype distribution of the domestic human strains was quite similar but not identical to the serotypes found in Finnish cattle and broilers. In Finnish cattle, Pen 2 and Pen 4-complex are the most prevalent serotypes followed by Pen 12, while Pen 1,44 has also been reported [Reference Hakkinen, Heiska and Hänninen17]. Pen 6,7 is rarely observed in cattle [Reference Hakkinen, Heiska and Hänninen17] but has been reported as the predominant serotype in Finnish poultry [Reference Perko-Mäkelä16]. Pen 12, Pen 4 complex and Pen 27 strains have also been found in poultry [Reference Perko-Mäkelä16]. Serotypes Pen 3 and Pen 37, that had the strongest association with imported infections, have not been reported in Finnish food-production animals. Although Campylobacter infections in humans and the prevalence of Campylobacter strains in broiler flocks and cattle follow the same seasonal pattern, it is not clear how much these animals contribute to human illness. The temporal overlap in serotypes and genotypes found in Finnish patients and chicken flocks at slaughter, suggest common environmental sources for both human infection and flock contamination [Reference Kärenlampi23]. An association between C. jejuni isolates from cattle, chicken and humans has been shown in other countries in studies exploiting serotyping and different genotyping methods and suggests a common environmental source of infection [Reference Schouls24–Reference Nielsen26].
In conclusion, >40% of the C. jejuni infections in Finland seem to be domestically acquired, and the number of domestic infections is highest in July and August. The serotype distribution of strains isolated from domestic and travel-associated infections differed. The demographic and geographic distributions of the serotypes of the domestic strains were serotype specific. Some serotypes were evenly distributed, whereas others varied both demographically and geographically. Both age-related acquired immunity and age- or area-related infection sources may play a role but the exact factors behind these differences remain to be determined.
ACKNOWLEDGEMENTS
We are grateful to the clinical microbiology laboratories for collection of isolates. The postgraduate studies of U.-M. Nakari were funded by the Finnish Graduate School on Applied Bioscience: Bioengineering, Food & Nutrition, Environment.
DECLARATION OF INTEREST
None.





