INTRODUCTION
There is abundant evidence of Myobacterium tuberculosis transmission among persons living in the same household, in geriatric centres and correctional facilities, as well as in educational centres and the workplace [Reference Bran1–6]. Tuberculosis (TB) outbreaks attributed to sporadic contacts have also been described, similarly those that have occurred on some forms of public transport [Reference Driver7, Reference Kenyon8]. Molecular epidemiological studies of M. tuberculosis isolates have shown clusters of cases that had not been detected by traditional epidemiological methods [Reference Small9–Reference Diel12], leading to the suspicion that a considerable proportion of infections go unrecognized in contact studies.
Between 2004 and May 2006, five cases of TB were diagnosed in young people aged 19–23 years in a municipality of 3300 inhabitants in Navarre, Spain. The mean annual incidence of TB in the 15–24 years age group in 2004–2006 was 4·5 cases/1000 population, compared to 0·15/1000 population in the same age group in the region of Navarre [Reference Castilla13].
Conventional contact tracing was carried out after the diagnosis of each case [14]. All close contacts and many occasional contacts were studied, including a total of 103 family members, friends, schoolmates and workmates. However, none of the cases met criteria for inclusion in contact studies of a previous case. Consequently, an epidemiological study was conducted to determine if this situation constituted an outbreak, to identify possible sources of infection, and to implement control measures.
METHODS
Contact investigation
The five cases of TB were interviewed to establish the places they had frequented for school, work and leisure time activities. Because these five cases constituted a very high incidence of TB in young persons, although no close relation had been found among the cases, it was decided to expand the contact study to persons in the same age groups who lived in the village according to the municipal census records. Local authorities and the board of the regional programme for TB control approved technical and ethical aspects of this investigation. All contact with people was established via the primary healthcare centre. Only participants who gave informed consent were included, and authorization was requested from the parents of minors.
The first step was to summon all those born between 1981 and 1990 (n=373) to a medical consultation to rule out the presence of symptoms, conduct an epidemiological survey and administer a tuberculin skin test (TST). In view of preliminary results, tuberculin screening was expanded to include those born between 1976 and 1980 (n=226) and between 1991 and 1995 (n=152).
The surveys were conducted before the TST results were known, and included sociodemographic variables (age, sex, home address and occupation), disease-related variables [previous diagnosis of TB, known contact with a case, vaccination with Bacillus Calmette-Guérin (BCG), TST, chemoprophylaxis] and public places frequented, including the 12 bars in the village, sports facilities, places where friends gathered and the school of music. Close contact was defined as an exposure to a known TB case for ⩾6 h per week, and occasional contact as contact for <6 h per week.
All persons lacking documentation of a previous positive test were advised to have the TST administered by the Mantoux method, which uses 2 IU of the RT-23 strain of purified protein derivative (PPD). The results were read by trained nurses. An induration of ⩾5 mm at 48–72 h was considered positive [14]. All those born between 1981 and 1990 that were tuberculin negative were advised to repeat the test at least 2 months after the diagnosis of the last potentially contagious case in the village. Few people in the other birth cohorts needed to repeat the test because the first tuberculin test was administered after this period had already elapsed. Tuberculin converters were defined as subjects with a previous negative test that had an induration at least 5 mm larger than that found in another test administered in the previous 2 years [14].
All persons who had a positive TST were investigated for the presence of symptoms of disease and underwent a radiological study of the chest. Suspected cases of TB disease were referred to a specialist for evaluation, and all others in this group were advised to undergo a 6-month course of chemoprophylaxis with isoniazid.
Study of TB cases
TB cases were considered to be those in whom M. tuberculosis was identified from culture, acid-fast bacilli were detected or, if both tests were negative, those meeting both clinical and radiological criteria for TB and receiving standard treatment. Cases in which acid-fast bacilli could be visualized in a sputum smear were considered infectious.
All M. tuberculosis isolates were compared using randomly amplified polymorphic DNA (RAPD) techniques following the method described by Yates et al. [Reference Yates, Drobniewski and Wilson15]. This method uses simple DNA extraction followed by a polymerase chain reaction (PCR) step involving a single primer. Restriction enzyme analysis was performed when the patterns obtained from the PCR products were indistinguishable, especially when only single similar-sized bands were obtained [Reference Yates, Drobniewski and Wilson15].
Statistical analysis
To quantify the association between different variables and the prevalence of TB infection, odds ratios were calculated with their corresponding 95% confidence intervals. Proportions were compared using the χ2 test or Fisher's exact test. Following bivariate analysis, multivariate logistic regression analysis was performed to identify the predictive capacity of each variable.
RESULTS
TB cases
The study began in May 2006 after the diagnosis of five cases in which M. tuberculosis had been isolated. Four cases had pulmonary TB with positive acid-fast bacilli sputum-smear results, and in two of the cases >3 months had elapsed between symptom onset and physician consultation. Expansion of the contact study made it possible to detect another two cases that had a positive TST and were clinically and radiologically compatible with TB. In December 2006 another new case of TB was detected in a person who had visited the village but did not reside there. Only one of these three new cases had a positive culture, but all received standard anti-TB treatment. All six culture isolates had the same chromosomal DNA restriction pattern, but different from other culture isolates in the region.
All eight cases were born in Spain between 1982 and 1987, and had negative HIV tests; six were male and two female. In the epidemiological survey, all except two of the cases reported knowing and having been an occasional contact of case 1 in settings where leisure time activities took place during the period when that person could have been contagious, although they did not initially meet the criteria for inclusion in the close contact study. All the cases had frequented a bar that one of the previous infectious cases had also visited (Table 1). Since then, and up to November 2008, no new cases of TB related to this village were detected.
Table 1. Epidemiological description of the eight cases of tuberculosis (a small village in Spain, 2004–2006)
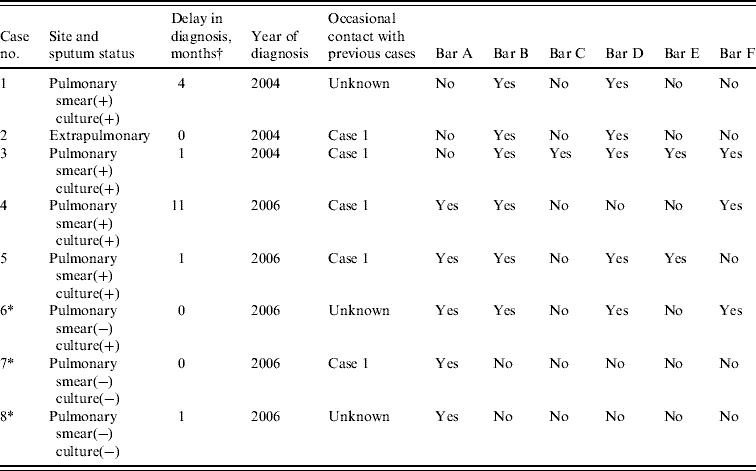
* Cases 6 and 7 were detected during the contact investigation, and case 8 did not reside in the village but was epidemiologically related with the outbreak.
† Time between symptom onset and initiation of treatment.
Prevalence of TB infection
A total of 692/751 young adults born between 1976 and 1995 were studied, and 59 were excluded: the five initial cases of TB, the 32 young people who did not usually reside in the village, and another 22 persons (2·9%) who either did not receive the TST or did not have it read (Fig. 1). In 90·6% of the persons studied it was possible to ascertain their BCG vaccination history by reviewing the vaccination card, by self-report, or by the presence of a vaccination mark. Some 24·0% of those born before 1991 were vaccinated, and none had been vaccinated after that year.
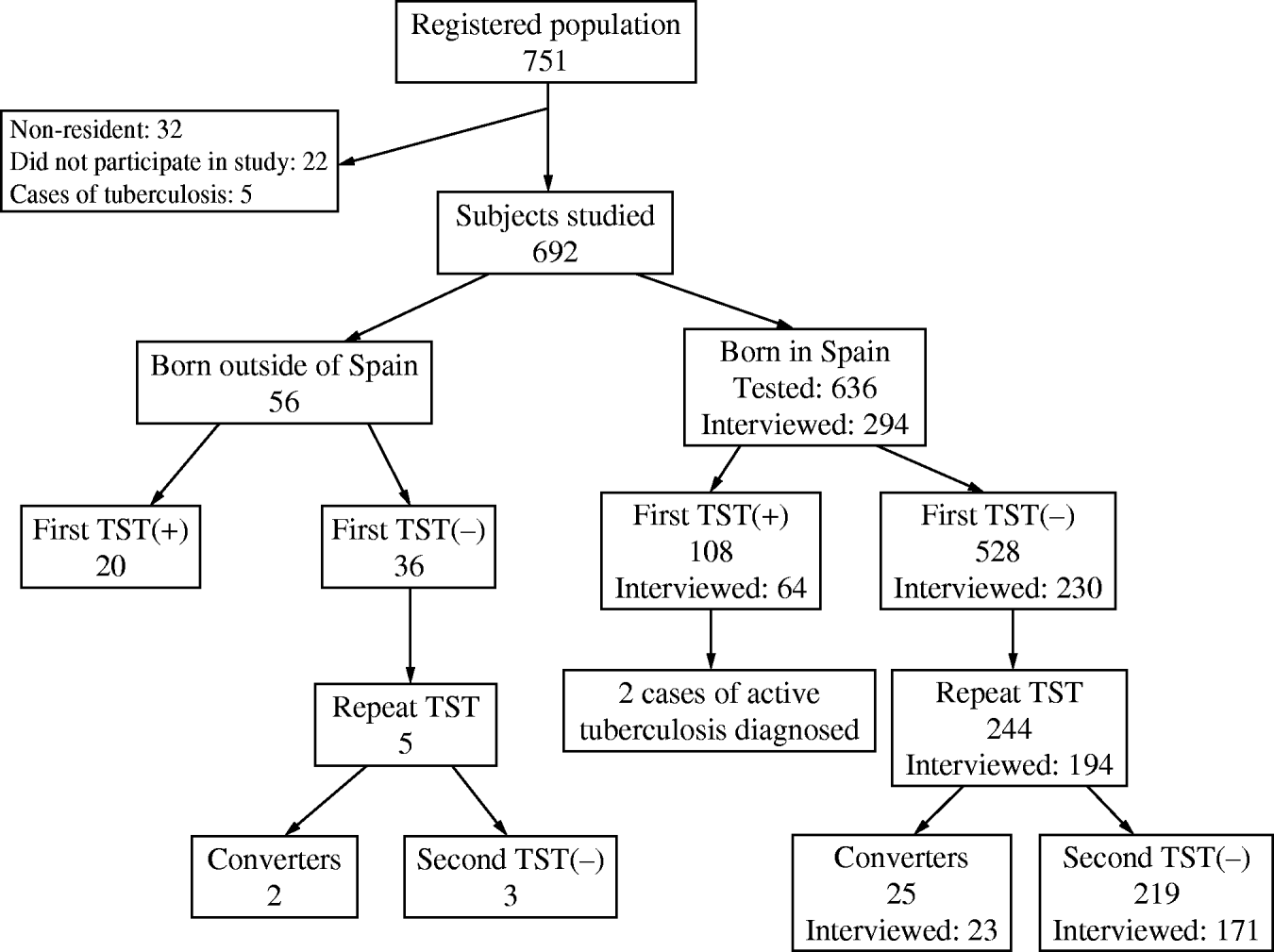
Fig. 1. Flowchart overview of the contact investigation in the resident population born between 1976 and 1995 (a small village in Spain, 2006). TST, Tuberculin skin test.
All these persons received the TST, except for 12 who had already been tested because they were contacts of one of the aforementioned cases and had had a positive result; these were directly classified as infected. Repetition of the tuberculin test 2 months after the detection of the last case of TB resulted in the diagnosis of 27 conversions (4·8%). In all, 18·5% of those studied were ultimately tuberculin positive, with differences between those born in Spain (17·0%) and those born in high-prevalence countries (35·8%, P=0·0019). In the subsequent analyses those born in countries other than Spain were excluded due to the difficulty of distinguishing between infections acquired in the country of origin and those acquired after visiting the village.
In those who were born in Spain, the final prevalence of TB infection was 45·9% in the cohorts born between 1985 and 1987 (19–21 years) and 29·7% in those born between 1981 and 1990 (16–25 years). Twenty-five tuberculin conversions were detected, all in persons born between 1978 and 1989 (17–28 years). The main excess of TB infection and all tuberculin conversions were observed in the 1978–1990 birth cohorts (Table 2). In those whose TST was initially negative and who repeated the test, the incidence of conversions was 17·5% in those vaccinated and 9·9% in those who were not vaccinated (P=0·1755). With a cut-off point of ⩾10 mm, these percentages were 15·0% and 8·2% (P=0·2270), and with a cut-off point of ⩾15 mm, they were 2·5% and 3·5% (P=1), respectively. About 28% of the converters had a history of BCG vaccination, but only one had an induration of <10 mm (Table 3).
Table 2. Status of tuberculin skin tests in the 1976–1995 birth cohorts (a small village in Spain, 2006)
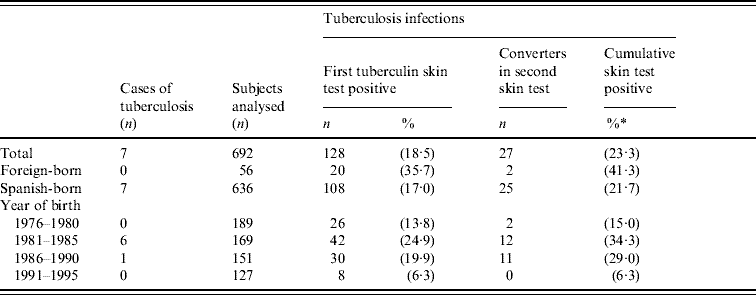
* Cumulative percentage of tuberculin skin tests positive after the first and second tests among subjects analysed.
Table 3. Results of the second tuberculin skin test (TST) in Spanish-born persons with negative first test, 1976–1990 birth cohortsFootnote * (a small village in Spain, 2006)
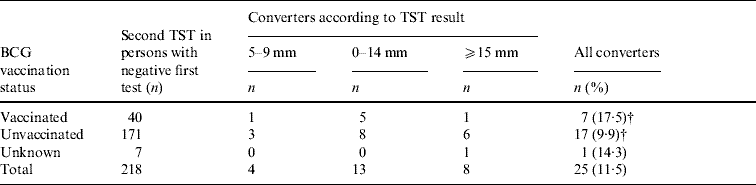
BCG, Bacillus Calmette-Guérin.
* None of those born after 1990 was vaccinated or was a converter.
† Comparison of percentages of converters between vaccinated and unvaccinated persons (P=0·1755).
Factors associated with TB infection
The survey included 294 residents of the village born in Spain between 1981 and 1990. None reported a history of TB or contact with other cases before the appearance of the identified cases. Some 8·5% had been close contacts (family or friends) of one of the infectious cases, and another 3·7% had work-related contacts, which was the reason they had been included in the corresponding contact studies. About 33·3% reported having sporadic contacts with one of the infectious cases, generally due to having met them in bars, although they had not been included in the contact studies. Some 91·2% of those surveyed reported frequenting a bar in the village, and 90·1% frequented at least one of the six bars mentioned by the cases.
The prevalence of TB infection was higher in men than in women (41·1% vs. 19·0%, P<0·0001). There were no statistically significant differences between sexes in having been a contact of a TB case or in having frequented bars, with the exceptions that bar A had been frequented more often by men (63·4% vs. 36·6%, P<0·0001) and bars B and D by women, and that men reported contact with case 4 in greater proportion than women (71·9% vs. 28·1%, P<0·0001).
In the bivariate analysis, both close contacts and occasional contacts of case 4 had a significantly higher prevalence of TB infection than those not reporting contact with that case. The occasional contacts of case 5 also presented a higher prevalence of TB infection, as did those who frequented bars A and F (Table 4). All these associations were maintained in the multivariate analyses, although due to the collinearity between being an occasional contact of some cases and visiting certain bars, both types of variables were evaluated in separate models (Table 5).
Table 4. Prevalence of tuberculosis infection and incidence of tuberculin conversion in study participants in the 1981–1990 birth cohorts (a small village in Spain, 2006)
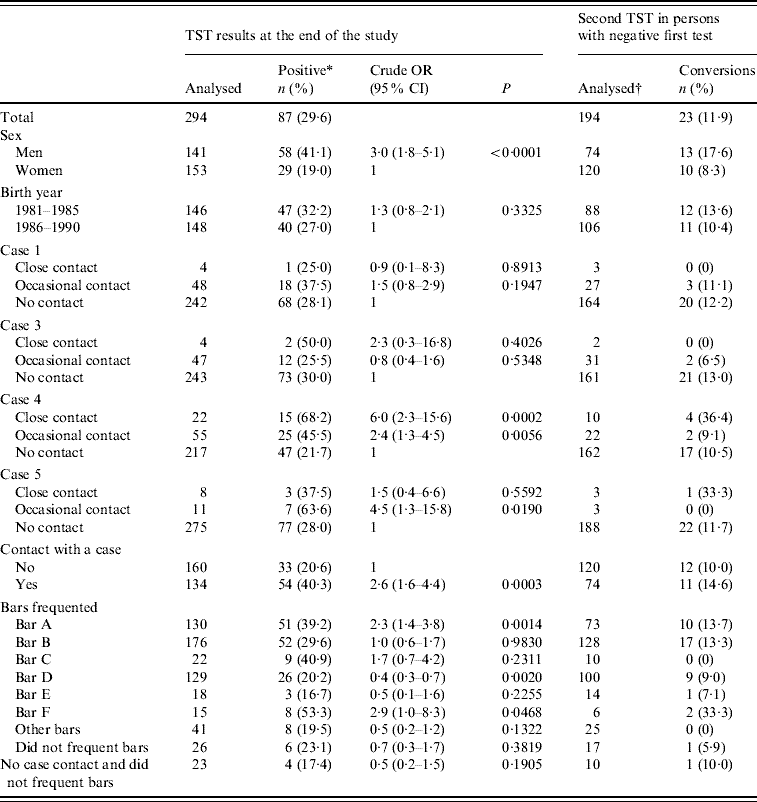
OR, Odds ratio; CI, confidence interval; TST, tuberculin skin test.
* Includes conversions.
† Thirty-six cases were negative in the first test and did not repeat it.
Table 5. Results of the multivariate analysis of variables associated with the prevalence of tuberculosis infection (a small village in Spain, 2006)
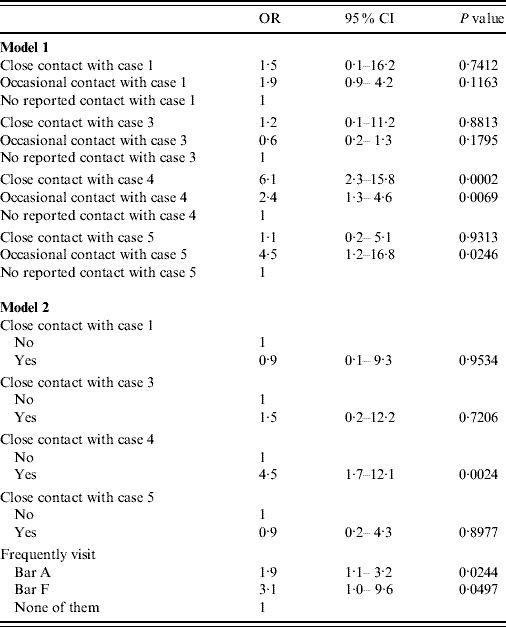
OR, Odds ratio; CI, confidence interval.
Close contact among friends, and persons who lived, worked or went to school together could explain only 24·1% of the TB infections detected, and 21·7% of the tuberculin conversions. Sporadic contact with a case could explain another 37·9% of the infections and 26·1% of the conversions. Having frequented the same bars as the infectious cases, even though they had no personal acquaintance, explained an additional 33·3% of the infections and another 47·8% of the conversions (Table 6).
Table 6. Distribution of young persons surveyed who were diagnosed with tuberculosis infection and tuberculin conversion, according to type of contact they had with infectious tuberculosis cases (a small village in Spain, 2006)
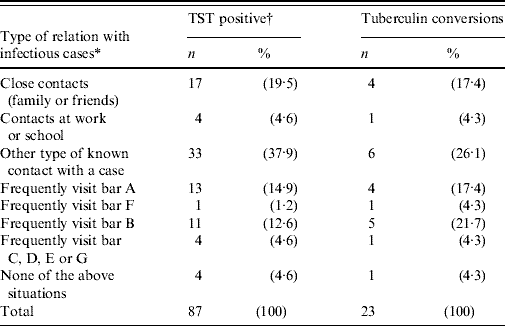
TST, Tuberculin skin test.
* A single type of relation was prioritized for each individual, following the order shown in the table.
† Includes tuberculin converters.
DISCUSSION
The present study demonstrates the existence of a TB outbreak with six cases confirmed by identical strains and another two epidemiologically associated cases, all in persons who resided in or had visited the same village. The cases in this cluster apparently had no close relationship, but all of them frequented some of the same bars in the village. To control the outbreak, a special intervention was carried out by expanding the contact study to 20 complete birth cohorts of young people in the village. We have found no precedents for expanding the study of contacts of TB cases to the whole population in a particular age group in a municipality. On this occasion this strategy was very well accepted by the population and allowed early detection of two additional cases of TB, 27 tuberculin converters and an excess of latent tuberculosis infections (LTI) that had not been detected in studies of the contacts around each case. With our study, we were able to provide early treatment for the new cases of TB and LTI, as fundamental measures to control the outbreak. The transmission probably involved family and work settings and various bars, and as the results show, limiting the outbreak and contact study to just some of these sites would have been insufficient. Moreover, it would be more difficult to assess and recruit the people that had visited each specific bar.
Most of the population studied and most of the converters did not have a history of BCG vaccination and only one of the vaccinated converters had an induration of <10 mm, which reduces the probability of false conversions due to the booster effect of tuberculin in vaccinated persons. False boostering from exposure to non-TB mycobacteria is unlikely because they are not common in that environment, and a high proportion of converters had >10 mm induration.
The results support the hypothesis that transmission was probably produced in settings around cases 1, 4 and 5. Delays in the diagnosis and initiation of effective treatment, as occurred in cases 1 and 4, have been reported to increase transmission of M. tuberculosis [Reference MacIntyre16–Reference Kettunen18]. Independently of the close contacts of the cases, there is strong evidence of transmission in bars A and F, although we cannot rule out the role of other bars or places, even though they were not significantly associated with a high prevalence of TB infection.
To explain this outbreak, two different times of transmission of M. tuberculosis were postulated. The first would have occurred between 2003 and 2004, when case 1 would have been the main source of infection, and cases 2, 3, and even 4, 5 and 7 could have acquired the infection. Case 3 could have contributed to the transmission, and some bars such as bar B, could have been the places of contagion. The second time of transmission would have occurred between 2005 and 2006. The primary source would have been case 4, although cases 5–7 could also have contributed. Bar A would appear to have played an important role. This bar was frequented by case 4 during the months in which he was symptomatic, and it was a poorly ventilated space, where a large number of young people congregated in close proximity. Other bars, such as C and F, could also have been places where infections were produced.
Several studies have described TB outbreaks associated with a single bar [Reference Bolam19, Reference Vidal-Alaball, Hayes and Jones20], or another place visited by a case of contagious TB [Reference Bock21–Reference Pettit25], but our study goes further by linking various bars and other types of exposures to the same outbreak. TB propagation can take a complex form when it is produced in more than one setting.
Men and women frequented different places which partly explains the different prevalence of infections. Moreover, men and women who had frequented the same bar differed greatly in the number of hours a week they spent there. Young people of similar ages tend to congregate in leisure settings which explains why the highest rates of LTI prevalence and 80% of the conversions were detected in people aged 19–25 years, an age range that included all the potentially contagious cases of TB. This study shows the high potential for TB transmission when the circumstances are favourable. In leisure settings such as bars, contacts with a much larger number of persons can occur than in home or work settings, and the accumulation of many persons in closed environments can favour the transmission of TB. Conducting a contact study in public places where leisure activities take place is difficult, since there is no list of exposed persons. Yet another difficulty is produced when more than one place is involved, as appears to have occurred in our study.
Our results show that a considerable percentage of the episodes of contagion in young people may escape detection in studies of contacts with family members, friends, school friends and workmates. In this small community, expanding the contact study to the young population proved useful for the early detection of new cases and for the diagnosis and treatment of LTI.
ACKNOWLEDGEMENTS
We thank all professionals of the Primary Health Care Centre for their useful contribution to the early detection and control of the outbreak, and Kathy Fitch for editorial assistance. This study was supported in part by the Plan Nacional de I+D+I through the Instituto de Salud Carlos III (FIS PI061346).
DECLARATION OF INTEREST
None.









