INTRODUCTION
Enteric infectious diseases are complex, multifactorial conditions resulting from interactions between numerous factors including pathogen exposure, infectious dose, strain variation, environmental conditions, nutritional state and immune status [Reference Colwell1]. These factors are not specific to a single infectious agent and a diagnosis of enteric infection is usually made either when patients present as sporadic cases of gastroenteritis or as part of an outbreak. Asymptomatic enteric infections are only identified as part of research studies, in both case-control and cohort designs, and have been reported from both developed and developing countries [Reference Guerrant2–Reference Kamalratnam4]. Given the range of pathogens capable of enteric infection and replication within the gastrointestinal tract, the aetiology of both symptomatic and asymptomatic infection can be established only when appropriate laboratory facilities are available [Reference Simpson5]. In most developing countries, diagnostic capacity is limited to a few bacterial and parasitic pathogens, contributing to a large diagnostic gap in the identification of pathogens producing both symptomatic and asymptomatic infections [Reference Mathew6].
With repeated exposure to enteric pathogens at all ages, most enteric infections are asymptomatic, and the proportion that is asymptomatic increases beyond 2 years of age, possibly owing to the development of active immunity [Reference Velazquez7]. However, even in children aged <2 years, asymptomatic shedding can be detected for several days or weeks, with stools containing infectious viruses, bacteria, or protozoal cysts [Reference Kang8–Reference Kang11]. Persons with asymptomatic infections play an important role in the spread of many enteric pathogens, especially as they are unaware of their infection, take no special hygienic precautions and move normally from place to place [Reference de Wit12]. Asymptomatic infections in children may also have a major role to play in determination of nutritional status and susceptibility to other infections [Reference Steiner13].
In the present study, we highlight the use of molecular diagnostic tools to identify asymptomatic enteric infections, possibly resulting from a contamination or seeding event, in a birth cohort of children in a south Indian urban slum being followed for rotaviral infections. The identification of infections, which would not have been diagnosed by conventional techniques, in a large proportion of the paediatric population under study indicates the importance of the use of sensitive diagnostic techniques in studies where sequelae or protection from subsequent illness is an outcome measure.
METHODS
Study population
A birth cohort of 452 children, resident in an urban slum area in Vellore, was recruited between April 2002 and July 2003 and monitored for a total of 3 years to assess the protective effect of natural rotavirus infection on subsequent homotypic and heterotypic exposure. The study was based at three adjacent urban slums in Vellore: Ramnaickanpalayam, Chinnallapuram and Kaspa, covering 2·2 km2. Vellore municipality had an estimated population density of 1660 per km2 in 2001, but density in the slums is higher. Mean annual rainfall in this area is about 1050 mm, peaking between August and November. Water for drinking is supplied at intervals of 2–28 days by the municipality from overhead tanks. During summer, temperatures are above 40°C. The slum has temporary and permanent housing, poor water supply, large garbage dumps, open drains and poor roads. All households in the area were initially surveyed and information collected on the composition of the household in terms of residents, family structure, occupation and socioeconomic status. Following this baseline survey, all pregnant women were identified and visited at least once a week by the field worker. The family of each child born in the study area was approached for recruitment into the study cohort. Ethical approval was obtained for the study from the Christian Medical College Research Committee and recruitment depended on parents having given informed written consent.
During the follow-up period each child was visited at home, twice a week, to record any morbidity in the child, or any diarrhoea in the family. Faecal samples were collected every 2 weeks from each child whether or not diarrhoea was reported. Samples were also collected when a child had diarrhoea. Any child who developed diarrhoea was assessed clinically and treated appropriately.
Sample testing
All samples were screened for rotavirus using either of two ELISA kits, based on VP6 detection by a monoclonal or polyclonal antibody (Rotavirus screen EIA, cat. no. Rota 003, Microimmune Ltd, UK; Rota IDEIA, cat. no. K6020, Dako Ltd, Denmark). All diarrhoeal samples were screened by bacterial culture and serogrouping for enteropathogenic Escherichia coli, Salmonella spp., Shigella spp., Aeromonas and vibrios and by microscopy for protozoan and helminthic parasites, including coccidian parasites such as Cryptosporidium as previously described [Reference Simpson5].
Molecular testing for enteric viruses
The study protocol required testing of all ELISA positives by reverse transcription–polymerase chain reaction (RT–PCR) for confirmation of rotavirus by VP6 amplification and for genotyping of VP7 and VP4. In order to evaluate the relative sensitivities of the screening ELISA and RT-PCR for VP6, all 236 surveillance samples collected from asymptomatic children during a 2-week period in May–June 2003 were tested by both methods. When a high proportion of these samples were found to be positive, further studies were initiated.
A subset of 50 VP6 PCR-positive samples was genotyped by determination of VP7 and VP4 types. A subset of 28 surveillance samples and 10 diarrhoeal samples were also tested for noroviruses by screening with Ni and E3 primers as described below.
A 10% faecal suspension in balanced salt solution was used for nucleic acid extraction using guanidinium isothiocyanate/silica. Reverse transcription with random primers, VP6 detection and G- and P-typing semi-nested PCRs were performed as described previously [Reference Banerjee14–Reference Iturriza-Gomara16]. The random priming method has previously been shown to detect 10 000 particles/ml of 10% faecal suspension [Reference Iturriza-Gomara16]. The cDNA generated by reverse transcription was used directly for detecting genogroup II noroviruses using published methods [Reference Green17].
Additional data collection
When a unexpectedly high proportion of asymptomatic rotavirus infection was noted in the birth cohort, additional data was collected on rainfall and water supply, in addition to hospital laboratory-based data on other potentially waterborne infections, Cryptosporidium, cholera, hepatitis A and hepatitis E, presenting to the hospital during 2003, and compared with data for previous years during the period April–July.
RESULTS
A planned comparison of the sensitivity and specificity of ELISA and RT–PCR for VP6 resulted in the identification of a high rate of asymptomatic infections in the birth cohort under follow-up, during the period May–June 2003. The mean age of the cohort at this time was 6 months (s.d.=3·8 months). Of the 371 children who contributed to the period May–June 2003, 118 (31·8%) had been previously infected with rotavirus. Of a total of 1191 non-diarrhoeal samples from 371 children collected over the 2-month period of May–June 2003, 22 (1·9%) were positive by ELISA and of these, 362 samples were tested by RT–PCR and 147 (40·6%) were positive (Fig. 1). This peak in PCR detection in asymptomatic samples was confined to a 2-week period at the end of May and in early June, since subsequent samples from the same children were negative. During this 2-week period, there was a significantly higher detection of positive samples (127 PCR positives/236 PCR tested, 53·8%) (P<0·001) than during the period of May–June 2003, excluding the 2-week period (20 PCR positive/126 PCR tested, 15·9%). The detection rate by PCR was also higher (P<0·001) than in the previous follow-up period from the beginning of enrolment of the birth cohort for samples tested by PCR (119 PCR positive/585 PCR tested, 20·3%, from 1 April 2002 to 26 May 2003).

Fig. 1. Testing of non-diarrhoeal samples for rotavirus by reverse transcription–polymerase chain reaction (RT–PCR) for the VP6 gene in a birth cohort under surveillance (——). The numbers of samples negative (□) and positive (■) for rotavirus VP6 by RT–PCR in each month are shown as proportionate bars.
Genotyping of the VP7 gene was attempted for 50 samples in order to determine whether this was an outbreak due to a single strain, and VP7 types could determined for 29 samples. In 19 surveillance samples, a single G type was identified and in 10, there were multiple G types indicating mixed infection. The G types identified were G1 (n=25), G2 (n=7), G4 (n=1), G9 (n=4) and G10 (n=7). The VP4 types were P[8] and P[4] and P[11], the latter was found only in association with G10 strains. The proportion of multiple infections was significantly higher (P<0·001) in this 2-week period than in the period up to 26 May 2003, during when 66 of the 119 samples positive by VP6 PCR had G or P type determined and only 1·5% had multiple G or P types.
A total of 6/28 (21·4%) of surveillance samples tested for noroviruses during the period 27 May to 10 June 2003 were positive. Of these, two samples were also positive for rotaviruses. Other enteric pathogens were not tested in the surveillance samples.
During May–June 2003, there were 166 diarrhoeal samples of which three (1·8%) were positive for rotavirus antigen by EIA, and 34 (20·5%) were positive by VP6 PCR, this represented no difference in the 20·3% positivity by PCR that had been seen in diarrhoeal samples from the community tested from the start of follow-up for the birth cohort, where one of 10 diarrhoeal samples examined for noroviruses was positive. Bacteria identified in diarrhoeal samples included Salmonella C1 (n=1), Vibrio cholerae O1 (n=1), non-O1 vibrios (n=3), and Aeromonas (n=6).
Of the 70 diarrhoeal samples from the period 27 May to 10 June 2003, mixed enteric infections were found in 6·3% and of the 670 diarrhoeal samples from the period of follow-up prior to 27 May 2003, 3·5% had mixed infections (P=0·5).
There was a marked increase in the number of Cryptosporidium-associated cases of diarrhoea in the cohort in the period June–August 2003, with 14 infections during this period, whereas only six infections were documented in the period March 2002–May 2003 and seven infections during September 2003–March 2004. Earlier hospital-based data do not indicate any seasonality of cryptosporidial (data not shown) or rotaviral [Reference Banerjee14] infections in Vellore.
When the age of infected children in the cohort was studied, it was found that during the period of study, the median age of children with diarrhoea was 3·6 months, while the median age of children providing surveillance or asymptomatic samples was 6·3 months (Mann–Whitney U test P=0·0002). A similar pattern was seen in children infected with rotavirus where rotavirus diarrhoea was seen at a median age of 3·5 months but asymptomatic rotavirus infections were seen at a median age of 5·5 months (Mann–Whitney U test P=0·0004) as shown in Figure 2.

Fig. 2. The distribution of samples from asymptomatic (- - -) and symptomatic (——) children of different ages in a birth cohort tested for rotavirus by reverse transcription–polymerase chain reaction for the VP6 gene during the period April–July 2003. The median age of asymptomatic and symptomatic rotavirus-positive children are indicated by arrows.
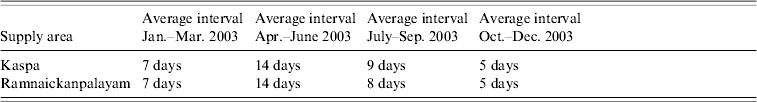
In order to identify a possible cause for the increased incidence of enteric pathogen shedding by the children, the water supply to the area was investigated. Most people in the area utilized the municipal water for drinking, with other water sources reserved for washing. The municipal water supply to the study families was from two main overhead tanks, Ramnaickanpalayam and Kaspa, which supply domestic and public stand pipes. Water supply is intermittent (Table) and during the 92-day period from May to July 2003, water was supplied a total of five times. Since the increased enteric pathogen positivity began after the third week of May 2003, it is interesting to note that water was pumped to the two areas on 21 and 24 May 2003.
Table. Drinking water supply to study areas
Additional data on disease due to other potential waterborne pathogens was collected at the referral hospital and showed no increase in numbers or proportion positive among samples tested for cholera during this time compared to the same months in the previous 3 years, but there were increases in cases of Cryptosporidium diarrhoea during June–August 2003 and hepatitis E cases during the following month from Vellore town (individual data not shown).
DISCUSSION
In the present study, we report the use of molecular techniques resulting in the identification of a possible contamination event resulting in widespread infection with a range of enteric pathogens which would have been undetected by conventional laboratory techniques. The only increased detection of pathogens associated with diarrhoea was with Cryptosporidium spp., but since this was associated with <10% of cases of diarrhoea, it was not recognized as an outbreak.
However, the use of RT–PCR permitted detection of rotaviruses in 40·6% of surveillance samples obtained during the period May–June 2003. This is much higher than the background prevalence of 5-10% positivity prior to and following this period of increased detection, as shown in Figure 1. In addition to multiple genotypes of rotavirus, noroviruses were also associated with asymptomatic infections during this period. The increased detection of enteric pathogens in the community could indicate a contamination event resulting in widespread infections. Since multiple pathogens and a significantly higher proportion of mixed infections were identified, a water source possibly contaminated with sewage would be the most likely source of infection. Resources for testing water samples for viral pathogens were not available, but previous studies in the area have found that all samples of drinking water are heavily contaminated with faecal coliforms, including those obtained from overhead tanks.
Since the detection of infection was by molecular-based tests applied to surveillance or asymptomatic samples, important questions to consider are the relevance of these results to the individual, to research, and to public health. At the level of the individual, a number of studies have reported the identification of enteric pathogens in asymptomatic individuals in both developed and developing countries [Reference Abiodun9–Reference Kang11, Reference Araya18–Reference Hellard22]. Reports from southern India have identified enteric pathogens in up to 50% of controls [Reference Simpson5], although more recent studies from the region indicate that the level of asymptomatic excretion of enteric pathogens is declining [Reference Kang23]. Children with diarrhoea and children with rotaviral diarrhoea were significantly younger than children who submitted surveillance specimens (P=0·0002), and children with asymptomatic rotavirus infection (P=0·0004) respectively, supporting the contention that protection from maternal antibodies may decline rapidly in developing countries. Studies from other developing countries have shown even asymptomatic enteric infections can have a significant negative impact on subsequent growth and development [Reference Steiner13, Reference Steiner24]. It can be argued that the impact of reducing enteric infections should be measured taking into account the effects on both short-term consequences such as reduced diarrhoeal morbidity as well as the long-term effects on nutrition and development [Reference Lima and Guerrant25].
The reduction of the entry of enteric pathogens into the environment is key to lowering the levels of subsequent infection. Enteric infectious agents survive well under most environmental conditions, remaining in the environment where they can be transmitted directly through food or water or indirectly by contact with contaminated faeces, fomites such as equipment or mechanical vectors such as flies [Reference Binka26–Reference Prado29]. In the causation of disease, key factors include the number of organisms in the infecting inoculum, their viability and the susceptibility of the host. When most infections in children resulting from a contamination event are asymptomatic, it can be argued that the infectious dose must have been low, because of the lack of disease or that prior exposure to the pathogens or maternal antibody protection resulted in a protective immune response. In this study, diverse genotypes of rotaviruses and noroviruses were detected in asymptomatic infections, in addition to an increased incidence of cryptosporidial infections. Polymicrobial enteric infections have been previously reported mainly in outbreaks of disease [Reference Hellard22, Reference Nimri30], but may be a common unrecognized event when water or other sources of infection are contaminated. It is also important to note that children asymptomatically excreting virus may also act as a source of infection for other susceptible hosts [Reference Nwachuku and Gerba31], because their mobility is not restricted by illness.
In considering the implications of detecting the occurrence of asymptomatic infections in this study, ELISA was found to be an inappropriate technique for the detection of asymptomatic infection. Although ELISA is sensitive and specific for the detection of group A rotavirus gastroenteritis [Reference Kang15] and previous studies on rotavirus epidemiology have used this technique [Reference Velazquez7, Reference Haffejee and Moosa32] when assessing asymptomatic infection or protection from disease, a detailed and careful investigation of infection that requires detection of asymptomatic infection should have molecular methods for rotavirus screening.
In community-based studies examining infection, disease and protection, it is important to use appropriate tools to ensure that infections are not missed due to a lack of sensitivity of the assay. This paper highlights the fact that the increased sensitivity of molecular tools enhanced the ability to detect infections that would have been missed by conventional ELISA-based techniques or by examination of only symptomatic cases. Understanding the burden and transmission of both infection and disease are important to formulate intervention strategies to prevent enteric infection and their long-term consequences.
ACKNOWLEDGEMENTS
This work was supported by Wellcome Trust Trilateral Cooperative Initiative on Infectious Diseases grant 063144. We thank Dr G. Sridharan and Dr Mary V. Jesudason for providing hospital data on potentially waterborne viral and bacterial infections.
DECLARATION OF INTEREST
None.





