Introduction
Childhood pertussis vaccination with whole-cell pertussis or acellular pertussis vaccines reduced notified pertussis cases about 90%. The greatest burden of morbidity and mortality is found in lower and middle-income countries [1].
The WHO strongly recommends pertussis surveillance for all countries [2], and possible methods for surveillance have recently been summarised [Reference Guiso and Wirsing von König3]. Serosurveys are one of these methods, which have been used in various countries and their role in surveillance has recently been specifically reviewed [Reference Wilson4, Reference Barkoff5]. However, serosurveys usually depend on a dedicated and expensive study design for collecting the samples. As pertussis is a cyclical disease, vertical serosurveys also only reflect the circulation of the bacteria in the population at a given time point in a specific region, and the differences between trough years and peak years of a cycle can be relevant.
Preparing the plasma pool for the WHO reference material for pertussis serology [Reference Xing6], we screened about 1000 donors yearly in 2002, 2003 and 2005 for immunoglobulin G (IgG)-anti-pertussis toxin (PT) levels of >100 EU/ml. In 2002 and 2003, only 0.7% and 0.3%, respectively, of donors had high IgG-anti-PT levels, whereas in 2005 we found 3.5%. Notification data at that time were only available from eastern German states, such as Mecklenburg-Pomerania, and they corresponded to our findings with incidences of 6.8/100 000 in 2002, 8.7/100 000 in 2003 and 74.6/100 000 in 2005 [7].
Thus, we decided to test an easily available alternative to conventional seroepidemiological studies, and we followed a cohort of blood donors for 3 years to study whether this selected population could be helpful in performing vertical and/or horizontal serosurveys of pertussis in resource-limited areas.
Materials and methods
Blood samples
We screened a randomly selected cohort of blood donors for IgG-anti-PT during 3 years on every donation. Samples from blood donors were anonymised apart from their age and sex. By giving blood, donors had also consented to the use of their samples, and no samples were specifically taken for this study. All procedures for blood donation, sample processing and sample testing were done according to the legal requirements in Germany, and all samples were also tested for the absence of markers for hepatitis B, hepatitis C and HIV infections.
A total of 307 blood donors were regularly followed between 2014 and 2016 for a total of 426 person-years. Due to their more regular donation habits, 81% (m:250/f:57) of the donors were male. The age ranged between 19 and 69 years (5–95%: 21–62 years) and the mean age was 39.6 years (median 41.8 years).
We had two donations from 278 donors (90.5%), three donations from 245 donors (79.8%), four donations from 190 donors (61.9%), five donations from 135 donors (44.0%), six donations from 90 donors (29.3%), seven donations from 55 donors (17.9%), eight donations from 25 donors (8.1%) and nine donations from 15 donors (4.9%).
Enzyme-linked immunosorbent assay
IgG-anti-PT was measured by an in-house enzyme-linked immunosorbent assay using purified PT as an antigen [Reference Wirsing von König8]. Values were reported in International Units/ml. As from 2015, a commercial IgG-anti-PT assay (Euroimmun AG, Lübeck) was used and the comparability of the in-house method and the commercial assay was intensively validated [Reference Riffelmann, Wirsing von Koenig and Schmetz9]. A recent contact was assumed, when antibody levels at least doubled, if the primary antibody level was above 12 IU/ml (the minimal level of quantitation), or when they converted to >12 IU/ml from non-detectable levels. For statistical evaluations, levels above 335 IU/ml were calculated as 335 IU/ml, and antibody concentrations below the level of detection (2 IU/ml) were calculated as 1 IU/ml.
Statistical evaluation
IgG-anti-PT values were tabulated and data were described by basic statistics. Differences between sets of raw data were tested for significance by Wilcoxon rank-sum test. Differences of P < 0.05 were regarded as significant (SigmaStat 3.1; SigmaPlot® 12.0, Systat Software GmbH, Erkrath, Germany).
Results
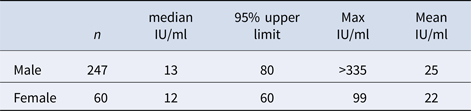
Table 1 shows the initial mean and median levels of IgG-anti-PT in various age groups, and no significant differences between the age groups were found (P > 0.1). Table 2 shows the initial mean and median level in male and female donors, and no significant differences between the groups were found (P > 0.1).
Table 1. Initial IgG-anti-PT levels in different age groups

Table 2. Initial IgG-anti-PT levels in male and female donors
Pertussis serosurveys are meant to indicate a recent response to pertussis antigens by either natural contact or vaccination. Using single serum cut-offs with 100, 62.5 and 40 IU/ml, we found that initially nine (2.9%), 22 (7.2%) and 54 (17.6%) of donors had IgG-anti-PT titres above the respective cut-offs. Four of the nine donors with initial titres of >100 IU/ml maintained high concentrations for at least 2 years (136/81; 108/155; 122/98; 282/184 IU/ml), indicating that high levels of IgG anti-PT may not always decrease during 12 months below lower cut-off levels.
The median interval between donations was 128 days and the mean interval 86, with a 95% confidence range between 49 and 404 days.
Table 3 shows the antibody levels during the study years (2014–2016), and both median and mean IgG-anti-PT concentrations remained rather stable throughout the study period (P > 0.1). Figure 1 shows boxplots of the IgG-anti-PT levels between 2014 and 2016. Differences in antibody levels were not significant between individual donations; however, in donors with seven (n = 55) or eight (n = 25) donations, antibody levels between the first donation and the seventh or eighth donation significantly decreased (P < 0.01 and P < 0.05, respectively).
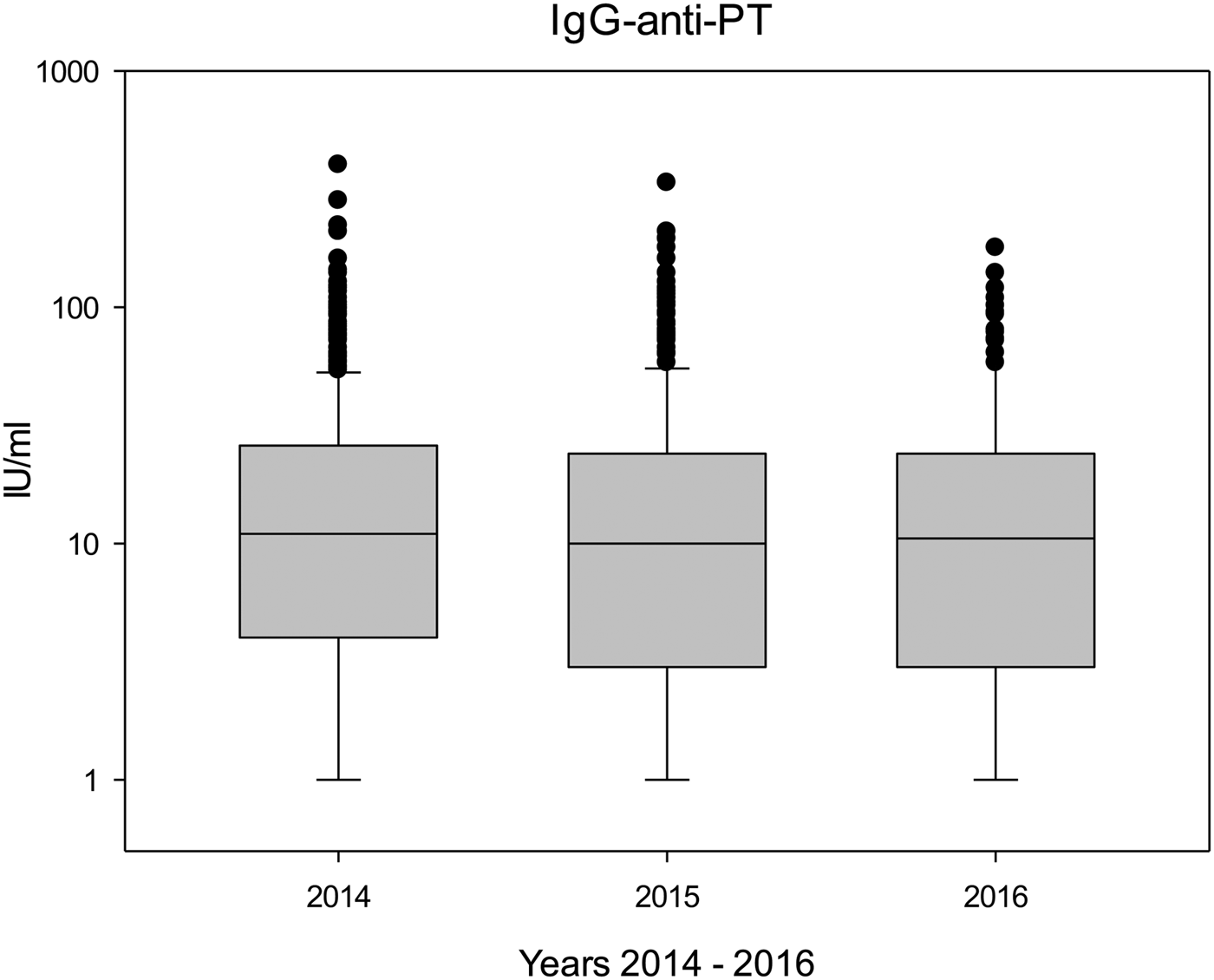
Fig. 1. Boxplots of IgG-anti-PT levels during the study period (2014: n = 566; 2015: n = 312; 2016: n = 132).
Table 3. Median and mean titres during the study period

During the observation period of 426 person-years, we observed six significant increases and two conversions, which resulted in an estimate of 1878 contacts/100.000 person-years (1.9%). One of the conversions (<2 to 28 IU/ml) would not have qualified as a suspected contact when usual cut-offs for single serum serology (>40 IU/ml) were applied.
Discussion
In contrast to seroepidemiological studies for other infectious diseases, pertussis serosurveys are used as a marker for recent contacts to Bordetella pertussis antigens such as PT in a given population. For estimating those recent contacts by a single sample, cut-offs had to be established. This was achieved by Dutch serosurveys carried out repetitively between the 1990s and 2007 [Reference de Melker10–Reference de Greeff12], by EU-wide studies [Reference Pebody13] and by analysing a big US serum collection [Reference Baughman14]. From these studies, cut-offs of 100–125 IU/ml IgG-anti-PT were suggested for diagnosing recent contacts, and 62.5 IU/ml for not recent contacts [Reference de Melker11, Reference de Greeff12], as well as 40 IU/ml as a lower limit for triggering additional tests in clinical diagnostics [Reference Guiso15]. These cut-offs have been used with slight variations in many other serosurveys worldwide. Seroepidemiology is estimating the frequency of immune responses as a marker for the circulation of B. pertussis in a population at a given time point and in a certain region. This method does not need symptomatic patients and only requires a standardised laboratory technique, which is commercially available [Reference Riffelmann, Wirsing von Koenig and Schmetz9]. Consequently, seroepidemiological studies of pertussis have been reported from many places of the world [Reference Koh16, Reference Fallo17].
In the EU, a big seroepidemiology study was performed with samples collected between 1995 and 1998 [Reference Pebody13]. From former West Germany, 1369 sera were sampled in 1995 among age groups 20 to >65 and 3.4% (47) of these contained IgG-anti-PT ⩾125 IU/ml. From former East Germany, only 122 samples were available, and 1.6% contained high IgG-anti-PT. When using the 125 IU/ml cut-off for our sample, 7/307 of donors (2.3%) initially had levels ⩾125 IU/ml.
Pertussis was a notifiable disease in former East Germany, and it continued to be notifiable since the reunification of Germany [18]. In 2013, pertussis was made notifiable also for former West German states, and the reported incidence rates per 100 000 of cases that fulfilled the reference definition in former West Germany were 14 (2014), 10 (2015) and 10 (2016) [Reference Hellenbrand19]. These notification data correspond nicely to our findings in the blood donors (Fig. 1), but also to the decrease between 2014 and 2015/2016 in donors with many donations.
However, seroepidemiology for pertussis also has limitations: it is not possible to distinguish between antibodies induced by natural contact or by vaccination, and if adolescent and/or adult vaccination programmes are existing, the interpretation of seroepidemiology should consider the individual vaccination status. Due to our strict anonymisation criteria, we had no information about vaccination history of the donors. Pertussis vaccination among adults during the precedent 10 years was assessed by a survey performed between 2008 and 2011 [Reference Poethko-Müller and Schmitz20]. Here, 12.5% coverage was found, ranging between 28.4 in the age group 18–29 and 7.5% in the age group 60–69. Adolescent vaccination was recommended in Germany in 2000 for adolescents aged 11–18, and in 2008, it was recommended that all adults receive one dose of Tdap. A more recent study performed in 2012–2013 found that only 7.6% of the overall adult population had had a pertussis vaccination during the last decade [Reference Bödeker21]. As a consequence, we would assume that our data were not intensively biased by recent Tdap vaccination.
Sampling can introduce another bias in respect to age groups and region [Reference Guiso and Wirsing von König3]. Our sample was restricted to adults in Krefeld, a city in North Rhine-Westphalia on the western border of Germany close to the Netherlands, and so our data reflect the seroepidemiology of pertussis only in this area.
Irrespective of these limitations, serosurveys in blood donors offer a simple method for estimating recent contacts to B. pertussis antigens, and our data show that they are vertically and horizontally applicable. As blood donation services are widespread in almost all countries, this simple method may produce estimates of pertussis circulation in those parts of the world, where data on pertussis circulation and incidence are currently not available.
Financial support
This study was supported by a grant from the ‘Robert-Koch-Institut’ (Federal Institute for infectious and non-infectious diseases) as part of the ‘Konsiliarlabor Pertussis’.
Conflict of interest
None.







