INTRODUCTION
Pertussis (whooping cough) is an acute respiratory infection caused by the bacterium Bordetella pertussis that can result in severe complications including pneumonia and apnoea which can lead to death, particularly in unimmunized infants. Despite the availability of vaccines since 1953, and various alterations to the National Immunization Program (NIP) in Australia over the last 10 years, control of pertussis continues to be a challenge in Australia with epidemics occurring every 3–4 years [Reference Quinn and McIntyre1]. Even though Australia has a pertussis immunization programme including a primary schedule for infants at ages 2, 4 and 6 months followed by a pre-school booster at age 4 years and an adolescent booster dose at age 14 years, almost 35 000 notifications for pertussis were reported in Australia in 2010. Reports of notification rates in Australia indicate that South Australia (SA) had the highest notification rate (448 notifications/100 000 population) compared to the national average of 154 notifications/100 000 population in 2010 [2]. While the significantly higher notification rates in SA may reflect differences in pertussis awareness, detection and notification, this provided a unique opportunity to examine the epidemiology of pertussis during an epidemic and provide valuable information to assist with prevention and management strategies.
METHODS
B. pertussis infection has been a notifiable disease in Australia since 1991. To assess the impact and epidemiology of pertussis infections, data collected by SA Health as part of the National Notifiable Diseases Surveillance System were analysed to review the epidemiology of the disease in a defined 18-month period. All cases in this series met national pertussis notification criteria with the majority (>99%) meeting criteria for a confirmed case [3]. Of the confirmed cases included in this review, 25% met laboratory definitive criteria (PCR positive) and 75% met laboratory suggestive evidence criteria (IgA + clinical evidence). The 17 probable cases included had clinical evidence of pertussis (coughing illness lasting ⩾2 weeks and either paroxysms of cough, inspiratory whoop or post-tussive vomiting). Anonymized data from cases notified during the review period of 1 July 2008 to 30 December 2009 including notification date, onset of illness date, age, sex, ethnicity, hospitalization (routinely followed up for all children aged <6 years), and immunization status including date administered and source of evidence [Australian Childhood Immunization Register (ACIR), GP/Medical record or parental report were reviewed]. Where no date of administration was available, immunization data were considered unknown.
Proportions of notifications and age-specific notification rates and hospitalization rates were calculated for defined age groups (<1, 1–3, 4–13, 14–24, 25–34, 35–44, 45–54, 55–64, 65–74 and ⩾75 years). Denominator data for rate calculations were obtained from the Australian Bureau of Statistics (ABS) population estimates [4]. To determine risks associated with hospitalization, notified cases requiring hospitalization were compared with case notifications reporting nil or unknown hospitalization. An assumption was made that hospitalized cases experienced more severe illness than non-hospitalized cases. χ2 testing was performed to test differences in proportions and risk factors associated with hospitalization and relative risk (RR) ratios [and 95% confidence interval (CI)] were calculated to determine the magnitude of risk.
The study was approved by the Children, Youth and Women's Health Service Human Research Ethics Committee.
RESULTS
SA notifications
From a population of about 1·60 million in SA, 6230 cases of pertussis were notified to SA Health between 1 July 2008 and 30 December 2009. For this 18-month review period, that reflects an overall notification rate of 383/100 000, or almost 0·4% of the population experiencing an episode of pertussis infection during the review period.
Seasonality
Pertussis notifications increased over the duration of the review period. The number of pertussis notifications reporting illness onset date between July and December 2009 were 2·7 times that for the same period in 2008 (3169 vs. 1180). Similarly, the number of pertussis notifications which reported hospitalization, were 1·7 times more than that reported for the same period in 2008. Overall the distribution of pertussis notifications showed about 200–250 notifications per month during August 2008–March 2009, followed by a steady increase in notifications per month until November 2009. This increase may be a result of several factors including increased awareness and testing (this period overlapped the influenza pandemic), more sensitive diagnostic methods, or a true increase in incidence. Regardless, the distribution of pertussis in SA by month of onset displayed an increasing trend in notification numbers and hospitalizations; however the proportion of notifications reporting hospitalization did not increase (Fig. 1). Although seasonality may be somewhat of a factor for infections spread by the respiratory route, for pertussis, any effect of seasonal variation is insignificant compared to the impact of the 3–4 year outbreak cycles for which this disease is known.
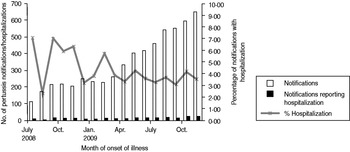
Fig. 1. Pertussis notifications, reported hospitalization and proportion reporting hospitalization in South Australia (July 2008–December 2009) by month of onset of illness.
Age- and gender-specific distribution
Of the total 6230 pertussis notifications reviewed for the period July 2008–December 2009, 41% were males and 59% females (2579/6230 vs. 3651/6230). Distribution by gender and age shows a higher proportion of notifications in females than males from the age of ∼14 years (2876/4670 vs. 1794/4670, P < 0·001); however, between the ages of 1 and 13 years, the proportion of pertussis notifications were roughly equal for both sexes (710/1428 vs. 718/1428), and for infants aged <1 year, a higher proportion of notifications received were for males (75/132 vs. 57/132, P = 0·027).
The majority (66%) of pertussis infections notified occurred in adults aged >24 years, with 25% (1546/6230) of all notifications being for individuals aged 24–44 years, a further 29% (1777/6230) were aged 45–64 years and 13% (811/6230) were aged ⩾65 years. Children aged 4–13 years accounted for about 20% (1238/6230) of the overall pertussis notifications and children aged <4 years accounted for just over 5% of the notifications, demonstrating that all age groups were susceptible to infection during this epidemic. Evaluation of notifications by age-specific notification and hospitalization rate suggest that the greatest risk of infection was for infants aged <1 year and children aged 4–13 years, and that a tenfold increased risk of hospitalization was apparent in infants aged <1 year compared to any other age group. The lowest risk of hospitalization (with age-specific rates <10/100 000) was seen in those aged 4–44 years (see Table 1).
Table 1. Age-specific pertussis notification and hospitalization rates/100 000 persons, South Australia, July 2008 to December 2009

* Per 100 000 persons/18 months.
† 2009 population estimates – Australian Bureau of Statistics.
While only 2% (132/6230) of all pertussis notifications received were for children aged <1 year, this group within the population is the most important when considering burden of disease and prevention of severe disease, as they accounted for 52 (21·6%) of the total 241 notifications recording hospitalization. Furthermore, 40% of notified cases occurring in infants aged <1 year required hospitalization.
Indigenous status
The ABS census June 2006 estimated the Indigenous population in SA to be about 1·7% of the total population in SA (28 055 Indigenous/1 567 888 total estimated residential population), although with an age structure which shows a higher proportion of infants aged <5 years compared to the Non-Indigenous population [5].
Overall, 83% (5159/6230) of pertussis notifications reported the case as Non-Indigenous, in 16% (1012/6230) the Indigenous status was unknown or not stated and <1% (59/6230) of notified cases were identified as Indigenous (59/6230). For those identified as Indigenous, there were no significant variations with respect to gender; 0·9% of notifications for males were identified as Indigenous vs. 1·0% of females; however, there were significant age variations in notifications of pertussis by Indigenous status. Only 2% of notified pertussis cases identified as ‘Not Indigenous’ were children aged <1 year, whereas 18% of case notifications identified as Indigenous were aged <1 year. In addition, while 67% of pertussis notifications for Non-Indigenous people were in adults aged ⩾25 years, for Indigenous people, only 45% of pertussis notifications were aged ⩾25 years. This may reflect the younger age structure and shorter life expectancy of the Indigenous population or may suggest less detection reporting of pertussis cases in Indigenous adults vs. Non-Indigenous adults. χ2 testing did not provide statistical evidence that Indigenous infants differed from Non-Indigenous infants with regard to pertussis immunization status. Additionally, small sample sizes limited meaningful inference on associations between Indigenous status and immunization history.
The proportion of notifications reported as requiring hospitalization was significantly higher (10/59, 16·9%) for those identified as Indigenous vs. those identified as Non-Indigenous (241/5159, 4·1%, P < 0·001). While the significant difference may suggest an increased severity of illness in Indigenous persons, it is possible that there are cultural differences between Indigenous and Non-Indigenous populations in their access to, timeliness of, and perceived importance for seeking medical advice leading to lower detection, testing and diagnosis of cases of milder pertussis in Indigenous persons and thereby overestimating the hospitalization proportion.
Immunization status
While only 21% (28/132) of infants aged <1 year with notified pertussis were younger than 2 months (the age at which the first dose of pertussis-containing vaccine is scheduled), 42% (56/132) were confirmed as having never received any pertussis-containing vaccination. Of the 56 notifications received with zero doses of pertussis-containing vaccine recorded according to ACIR, parental report or GP/medical record, 28 (50%) were considered too young for vaccination, 17 (30%) were age-appropriate to have received at least one dose, one infant was age-appropriate to have received two doses, and ten infants were age-appropriate to have received three doses. This may suggest that education on the importance of timely immunization and a greater understanding of the barriers to immunization may assist with the prevention of severe pertussis disease in the community.
The research findings demonstrate that children vaccinated according to the NIP are still susceptible to pertussis infection; at least 50% of children aged between 1 and 13 years who had a notified case of pertussis infection had received three or more doses of pertussis-containing vaccination according to current recommendations. Furthermore, 11% of infants aged <1 year with notified pertussis infection had also received a complete three-dose primary course of pertussis immunization (Fig. 2), which supports evidence that the current vaccine and schedule does not provide 100% efficacy.

Fig. 2. Proportions of pertussis notifications by age group and number of vaccine doses received prior to illness.
Of 4315 notifications for persons aged >20 years, 289 (6·71%) were reported by the notifying doctor as having received a pertussis vaccine. Evidence of vaccination, however, was only available for 212 (4·8%); the lack of a vaccination register for adults in Australia limits the ability to determine true coverage. It is likely that vaccine coverage in adults who were not vaccinated as part of the adolescent school programme (introduced in 2004 for 15- to 17-year-olds) is very low and may be a contributory factor to the transmissibility of pertussis and the epidemic.
Risk factors for hospitalization (Table 2)
Gender
For males and females respectively, 4·1% (106/2579) and 3·7% (135/3651) of pertussis notifications reported requirement for hospitalization (106/2579). This difference was not statistically significant for either all notifications in SA (P = 0·405) or for infants aged <1 year (P = 0·202).
Table 2. Risk factors for hospitalization in notified pertussis cases for infants in South Australia
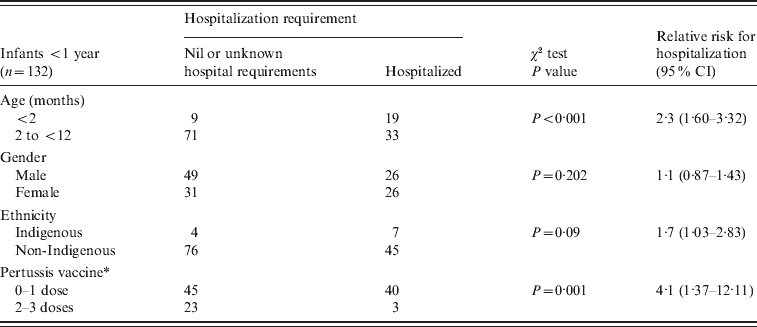
CI, Confidence interval.
* Those without evidence of vaccination were considered unknown and excluded in this analysis.
Age
For infants aged <1 year, a greater likelihood of hospitalization was observed for infants aged <2 months compared to those aged ⩾2 months (P < 0·001).
The risk of hospitalization with pertussis for infants aged <2 months was 2·3 times that of infants aged 2–12 months (95% CI 1·60–3·32).
Indigenous status
As a significant variation in age distribution of notifications between the Indigenous and Non-Indigenous populations was apparent, we compared the proportion requiring hospitalization to total notifications for children aged <1 year, the age group at most risk of severe disease.
Indigenous infants with pertussis were more likely to be hospitalized than Non-Indigenous infants; however, the case numbers are small for the Indigenous population. Of 11 pertussis cases notified as Indigenous infants aged <1 year, seven (63·6%) reported hospitalization. For Non-Indigenous infants aged <1 year, 105 notifications were received, of which 42 (40%) required hospitalization (P < 0·001). A higher risk of hospitalization for pertussis was associated with Indigenous ethnicity (RR 4·09, 95% CI 2·29–7·30). However, it is important to note, that the majority of notifications for Indigenous persons were for infants aged <1 year 11/59 (19%), vs. 105/5159 (2%) for Non-Indigenous notifications. If we consider only children aged <1 year to control for age variations, then risk of hospitalization for Indigenous compared to Non-Indigenous individuals is lower but still apparent (RR 1·71, 95% CI 1·03–2·83). χ2 testing for association between Indigenous ethnicity and hospitalization for infants aged <1 year suggested a trend (P = 0·086) but was not significant at α = 0·05 level. This may suggest a true increased risk of severe disease in Indigenous vs. Non-Indigenous infants, or may be the result of poorer detection and notification of milder pertussis for the Indigenous population.
Immunization status
A χ2 test demonstrated an association between hospitalization and immunization status (0 or 1 dose vs. 2 or 3 doses) for notifications in infants aged <1 year (P = 0·001). The relative risk of hospitalization for those with one or fewer immunizations was 4·1 times that for infants who had received two or more immunizations (95% CI 1·37–12·11). In addition, in notified cases, the risk of hospitalization for infants who had never received any immunization was 1·24 times that of infants who had received one dose (95% CI 0·76–2·01); however, this was not statistically significant (P = 0·375).
It is important to be aware that being aged <2 months was also associated with higher risk of hospitalization. As the first dose of pertussis vaccine is scheduled at about age 2 months, a high proportion of those having received zero doses of vaccine were also aged <2 months (28/56, 50%), therefore the hospitalization and immunization associations may be confounded by age.
A stratified analysis looking only at children who were aged 6–12 months also demonstrated that for infants (aged 6–12 months) who had received fewer than two doses of vaccine, the risk of hospitalization was 2·5 times that of infants (aged 6–12 months) who had received two or more doses of vaccine (95% CI 0·52–11·96). A χ2 test did not demonstrate significant association (P = 0·23) between number of doses of vaccine and hospitalization for this age cohort. However, the sample size for this analysis was very small (n = 36) with only 6/36 cases requiring hospitalization.
DISCUSSION
This review of pertussis cases notified in SA between 1 July 2008 and 30 December 2009 has demonstrated that the majority of pertussis infections in SA occur in the adult population, with more than 65% of notifications occurring in people aged >24 years. Infants, while only a small proportion (2%) of the overall notifications, were a significant proportion (22%) of the notified cases that were hospitalized and are an important group to consider when evaluating the overall impact or burden of pertussis in SA. Overall, gender-specific differences were noted with young male infants being notified for pertussis more frequently than females, but adult females being notified more often than males; however, there were no statistically significant gender variations associated with hospitalized cases. Potential for gender-specific variations with pertussis infections are not well understood, but hypotheses have included hormonal influences on severity of infection and greater exposure to young children with pertussis [Reference Edwards, Decker, Plotkin and Orenstein6]. Previous research in Australia has suggested that the incidence of pertussis is higher in females and may be a result of gender-specific differences such as likelihood of presenting for medical review [Reference Cagney7]. However, these theories would not explain an increased incidence in males in infancy. It will be important to validate and understand any gender-specific risks for pertussis infection that are dependent on age.
Review of age-specific rates suggest that infants aged <1 year, followed by children aged 4–13 years are the most important groups to target for reducing transmission as these groups had substantially higher notification rates than other age groups (Table 1). Age-specific hospitalization rates also indicate that infants aged <1 year are at highest risk of hospitalization from pertussis (at least tenfold higher than any other group), and therefore need to be highly considered in strategies for prevention of pertussis infection.
While complete data on immunization were not available, and was passively reported for adults, reasonably complete immunization data were available for the majority of infants aged <1 year (89%) and most of the notifications for children aged <3 years (80%). Immunization data analysis showed that only half of the infants who had not received any pertussis vaccination were too young (<62 days) according to the NIP, and 11/28 (39%) who were unvaccinated at onset of illness were age-appropriate to have received at least two doses of pertussis vaccine. This would suggest that earlier or more timely immunization may confer considerable direct protection against hospitalization with pertussis infections in infants and may reduce the burden on healthcare systems and significant distress to affected families. It is important to also highlight that 11% of pertussis cases notified for infants aged <1 year occurred in children who had received three doses of vaccine, confirming that pertussis vaccination according to the current schedule does not provide complete protection against pertussis infection. However, previous research has suggested, that pertussis vaccination is associated with reduced severity of disease [Reference Preziosi and Halloran8, Reference Juretko9].
The greatest rates of hospitalization were evident at the two extremes of life – newborns and the elderly. Almost 40% of the notifications for infants required hospitalization (i.e. 2/5 infants with confirmed pertussis required hospital admission). The age group with the second highest rates of hospitalization were those aged ⩾75 years, with 11·4% of the notifications reporting hospital admission. Despite these two age groups making up a very small proportion of the total number of notified pertussis cases (412/6230, 6·6%), about one third of all the notified cases reporting hospitalization (84/241, 34·9%) were either infants aged <1 year or adults aged ⩾75 years. This might suggest that improved pertussis booster vaccination uptake for elderly adults may be a further strategy for prevention of pertussis (both for the individual and for prevention of transmission to vulnerable grandchildren) and reduction of healthcare system costs in the SA population.
From this research, our Indigenous community appears at particular risk of hospitalization with pertussis, especially in infancy with where the relative risk of an Indigenous person with pertussis being hospitalized is 4·1 times that of a Non-Indigenous person (95% CI 2·29–7·30). This may be a result of less timely immunization [Reference Hull10], remote living conditions [Reference Kolos, Menzies and McIntyre11] or increased likelihood of transmission through larger household size. However, variations in age structure and issues such as incomplete identification of Indigenous status and probable underreporting of milder disease in the older Indigenous population may lead to an overestimation of risk ratios. Immunization coverage rates are high for both Non-Indigenous infants and Indigenous infants with 85% of Indigenous Infants and 92% of Non-Indigenous infants receiving three doses of pertussis-containing vaccine by age 12 months [Reference Hull10]. Age-stratified analysis on infants aged <1 year indicated that the increased relative risk of hospitalization for Indigenous infants remained, although somewhat lower in magnitude (RR 1·71, 95% CI 1·03–2·83). However more data, with a larger sample, more thorough immunization information and demographic information would be required to provide a better understanding of the increased risk of severe disease for the Indigenous community.
A limitation of this study regarding identification of risk factors for hospitalization is the lack of complete follow-up for this outcome for all notifications. However, all children aged <5 years are routinely followed up for this outcome and data are likely to be reliable for this group. Similarly, more thorough, accurate adolescent/adult immunization data would have strengthened any inferences that could be made on the relationships between immunization coverage, time interval between immunization and reporting of pertussis case and background immunization rates in adults; however, this level of follow-up was not possible with this research study. Data that were analysed for increased risk for hospitalization (for infants aged <1 year) demonstrated that at age <2 months, fewer than two doses of pertussis containing vaccine and Indigenous ethnicity were associated with hospitalization. Other factors that were not able to be examined in this study such as primary vs. secondary cases may also be important for severity of illness [Reference Nielsen, Hedegaard and Aaby12].
The data presented suggest that pertussis is a significant burden in the SA population and causes infections over the entire age spectrum. Improved protection for newborns and the elderly are vital for reducing severe pertussis disease and further research on vaccine efficacy against circulating B. pertussis is warranted to provide improved control of pertussis.
CONCLUSIONS/RECOMMENDATIONS
Prevention of pertussis disease requires control strategies at a number of levels. Direct protection is best afforded through the timely delivery of at least two doses of pertussis-containing vaccine followed by booster doses to maintain immune memory. As these data show, infants, primarily those too young to have the benefit of full vaccination, are most at risk of severe, life-threatening illness. Thus, as a strategy, individual vaccination alone is not enough to prevent severe illness and death from pertussis. To further protect susceptible infants, a range of strategies can be considered: reducing transmission in the general population through improved booster vaccination of adults, including couples planning a pregnancy, and targeted vaccination of household members domiciled with infants. Although the first dose of pertussis-containing vaccines are due to be delivered at age 2 months they can be delivered from age 6 weeks. The 6-week post-delivery check is an opportunity to administer all vaccines scheduled for age 2 months.
Research studies are currently assessing the value of delivering birth doses of pertussis vaccine and maternal immunization during pregnancy as a means of providing earlier protection to infants [Reference Wood, McIntyre, Marshall and Roberton13]. This research study shows that adults and adolescents are a significant reservoir for pertussis and potential source of further transmission to vulnerable infants. Additionally, vaccination according to the recommended schedule appears preventative for severe disease but does not always provide immunity against infection. Furthermore, only 30% of infants who were age-appropriate for receipt of a complete primary course of pertussis vaccination (three doses) had actually received three doses of pertussis vaccine, indicating there is further room for improved prevention of severe pertussis through timeliness of vaccination and education. A combination of strategies aimed at improving direct protection for newborns as well as minimizing community transmission and reducing spread of infection from close contacts of infants are required for prevention of severe pertussis disease.
DECLARATION OF INTEREST
Helen Marshall has been a member of vaccine advisory boards for GlaxoSmithKline (GSK) Biologicals and her institution has received funding for investigator-led research from GSK and Sanofi-Pasteur. She has received travel support from GSK Biologicals and CSL to present scientific data at international meetings.
ACKNOWLEDGEMENTS
Helen Marshall acknowledges the support of the National Health and Medical Research Council of Australia, Career Development Fellowship (1016272).






