INTRODUCTION
In developing countries, an estimated 10–15% of tuberculosis (TB) cases are children [Reference Murray, Styblo and Rouillon1, Reference Nelson and Wells2]. The control of childhood TB has not been given high priority, compared with the Directly Observed Therapy, Short-Course (DOTS) strategy for adults, partly due to the difficulty of diagnosing paediatric TB [Reference Nelson and Wells2, Reference Stark3]. However, young children have a higher risk of progression to severe disease, and consequently TB is a major cause of morbidity and mortality in children worldwide [Reference Nelson and Wells2, Reference Stark3].
Until recently, the tuberculin skin test (TST) has been the best available method to diagnose latent TB infection, although it has many limitations (e.g. low sensitivity in immunocompromised patients and cross-reactivity with hypersensitivity due to Bacille Calmette-Guérin (BCG) vaccinations and non-tuberculous mycobacterial (NTM) infections) [Reference Huebner, Schein and Bass4]. Newly developed interferon (IFN)-γ assays have high sensitivity and specificity in students and adults with active TB disease and offer a better correlation with the risk of infection in terms of intensity of exposure to Mycobacterium tuberculosis, regardless of BCG vaccination history [Reference Mori5–Reference Ferrara11]. However, little is known about its performance in children [12–Reference Tsiouris15], with the exception of a few studies using ELISPOT [Reference Liebeschuetz16–Reference Hill18].
The aim of this study was to assess the QuantiFERON-TB® Gold test (QFT) in young children aged ⩽5 years by comparing results of QFT with those of TST for household contacts of TB cases with varying infectivity.
MATERIALS AND METHODS
Subjects
The study subjects were children aged ⩽5 years who had been living in the same household with a newly diagnosed pulmonary TB patient for 1 month or more. The index cases were consecutively registered under the National TB Control Programme (NTP) between 5 September and 11 November, 2005, at Phnom Penh City and Battambang Province, Cambodia. To reconfirm the diagnosis of TB and smear positivity, we examined two sputum specimens for microscopic smear and culture in addition to the three sputum smear examinations routinely performed. Niacin test was performed for microbiological identification of all culture-positive strains. It is NTP policy to diagnose smear-negative TB based on the WHO guidelines [19]. We included children who were contacts of cases with smear-negative and culture-negative TB in this analysis to use them as part of the control group to compare with those who were contacts of cases with smear-positive TB.
The medical check-up for the subjects included chest radiography, TST, and 5 ml blood collection for QFT. To diagnose childhood TB, we followed the NTP guidelines [20] using a combination of clinically major and minor signs such as TST result, abnormal chest radiograph and symptoms, regardless of QFT results. We included children who were diagnosed with active TB in the analysis. No HIV tests or sputum examinations were conducted. A medical check-up with written informed consent by the parents or guardians was carried out between 16 and 28 January 2006, at least 9 weeks after final contact with the untreated index case. The research was approved by the National Ethics Committee for Health Research, Ministry of Health, Cambodia.
The Expanded Programme on Immunization in Cambodia encourages all children to be vaccinated with BCG at birth. The vaccine used is manufactured by State Serum Institute, Copenhagen, Denmark.
TST and whole-blood IFN-γ assay
For the TST, 0·1 ml of purified protein derivative (PPD) (Nippon BCG Manufacturing, Tokyo, Japan; equivalent to 2·5 TU of PPD-S) was injected intradermally into the volar aspect of the forearm. Two trained nurses independently measured the transverse induration diameter in millimetres after 72 h.
The QFT test was performed according to the manufacturer's instructions [21, 22]. Following 16–24 h of incubation of heparinized whole blood with ESAT-6, CFP-10, mitogen (for positive control), or saline (for nil control), plasma samples were frozen at −70°C and shipped to the Research Institute of Tuberculosis, Japan Anti-Tuberculosis Association, to determine the concentration of IFN-γ. A positive response of 0·35 IU/ml of IFN-γ was used as the cut-off point, the same as for adults [Reference Mori5, Reference Brock6, 21, 22]. An IFN-γ response of ⩽0·35 IU/ml of antigen and ⩽0·5 IU/ml of mitogen was defined as ‘indeterminate’ and excluded from the analysis [21, 22].
Statistical analysis
In the absence of a ‘gold standard’ for identifying latent TB infection, we examined the validity of QFT for diagnosing latent TB infection using a concordance (a proportion of cases with matching classifications and a κ-coefficient) of results between QFT and TST. We used the McNemar test on the same subgroup and a χ2 test on different subgroups to compare the TST and QFT results. Logistic regression models were used with SPSS version 13.0 (SPSS Inc., Chicago, IL, USA) to evaluate four factors related to the children (sex, age, BCG scar, and time interval between the diagnosis of the index case and the test), and smear positivity of the index case. We included all the five factors in the multivariate analyses, irrespective of univariate statistical significance, because each of them is a factor well-known to affect the TST result [Reference Rieder23–Reference Chadha, Jagannatha and Kumar26]. We judged a P value of <0·05 to be significant.
RESULTS
Characteristics of contacts and index cases
During the enrolment period, 289 smear-positive and 120 smear-negative TB cases were registered at the two study sites. Of these, 117 smear-positive cases and 47 smear-negative cases were living with 183 and 64 children, respectively aged <5 years of age at the time of enrolment. Twenty-eight children without written informed consent and two children without TST readings were excluded from the analysis. Consequently, a total of 217 children who were household contacts of 161 pulmonary TB cases were tested with both TST and QFT (Table 1). Twelve children (5·5%) were aged 5 years at the time of examination. BCG scars were found in 191 children (88%). Among the 217 children, 19 children were diagnosed with active TB, including pulmonary disease, cervical or hilar lymphadenitis in the contact investigation.
Table 1. Characteristics of children
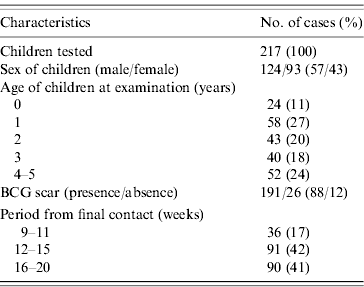
BCG, Bacille Calmette-Guérin.
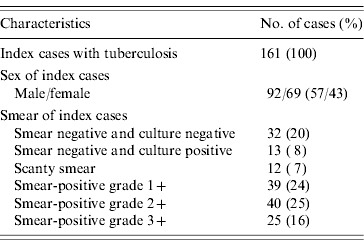
The characteristics of the index cases are listed in Table 2. Of the 161 index cases, there were 45 smear-negative cases (28%), with 32 testing negative for culture and 13 positive. Of the 117 smear-positive cases, 51 (32%) were scanty smear or smear-positive grade 1+, 40 (34%) grade 2+, and 25 (21%) grade 3+.
Table 2. Characteristics of index cases
Cut-off point of TST
The Figure presents a histogram of TST induration in millimetres among the children measured. It clearly indicates a bimodal shape at the centre of 10 mm; therefore, we considered being positive as a cut-off point of 10 mm TST induration. Of the 217 children, 48 (22%) had positive TST results.

Fig. Distribution of tuberculin skin test (TST) among children (n=217). □, Contacts with smear-positive TB; ■, contacts with smear-negative TB.
Comparison between TST and QFT results
Of the 217 children, 195 tested positive or negative with QFT, and 22 had indeterminate or missing test results. Of these 195 children, 47 [24%; 95% confidence interval (CI) 18·7–30·6] were TST positive, and 33 (17%; 95% CI 12·3–22·8) were QFT positive (Table 3). Of the TST-negative reactors, 3·4% (5/148) had a positive QFT. Among the 47 TST-positive reactors, 28 (60%) were QFT positive. The QFT-positive rate for those with 10–14 mm TST induration was 47%, while the rate for those with ⩾15 mm induration was 82%, significantly higher than the former case (Yates' χ2=4·35, P=0·037). Nine children had an indeterminate QFT due to a low IFN-γ response to mitogen, and 13 children had too little blood drawn to test for mitogen. Of the 19 children with active TB, 15 (79%) were TST positive and 10 (53%) were QFT positive.
Table 3. Comparison between TST induration and QFT results (%)
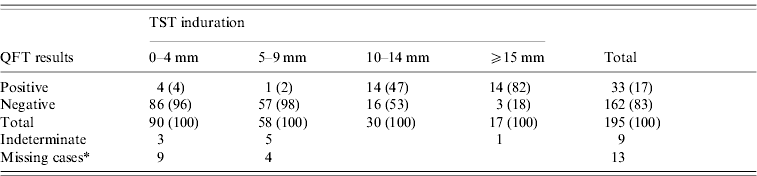
TST, Tuberculin skin test; QFT, QuantiFERON-TB® Gold test.
* Due to unsuccessful phlebotomy.
To assess the concordance between the two tests with varying risks of infection, we classified the child contacts into four subgroups: smear-negative with positive or negative culture, smear-positive grade 1+ including scanty smear, smear-positive grade 2+, and smear-positive grade 3+. As seen in Table 4, the overall positive rate of 24% for TST was significantly higher than that of 17% for QFT (P=0·007). For each category of contacts, the positive rate of TST was higher than that of QFT, although the difference was not statistically significant. An increase in positive rate for both QFT and TST with increasing smear positivity was clearly observed, with statistical significance for the linear trend in proportion (QFT: χ2=8·29, P=0·004; TST: χ2=7·53, P=0·006).
Table 4. Concordance rates and κ-coefficients by smear positivity of index cases

TST, Tuberculin skin test; QFT, QuantiFERON-TB® Gold test.
* Including scanty smear.
† P=0·004 in QFT and P=0·006 in TST positive rate for trend.
‡ P=0·007 with McNemar test for difference between QFT and TST positive rate.
Table 4 also presents the concordance rates and the κ-coefficients for the four groups. The concordance rates ranged from 0·84 to 0·91 among the groups and the overall concordance rate was 0·88. The overall κ-coefficient of 0·63 (95% CI 0·49–0·76) for the two tests was quite high, although the κ-coefficients among the groups ranged from 0·31 to 0·77.
Table 5 compares the results of the two tests by age of the children. In each age group, the TST-positive rate was higher than or equal to the QFT-positive rate, although the difference was not statistically significant. No statistical significance for a linear trend with age was found with either QFT or TST (QFT: χ2=0·001, P=0·97; TST: χ2=1·49, P=0·22). The κ-coefficients ranged from 0·40 to 0·83 among the age groups, and that of 0·40 was the lowest in the group aged <1 year.
Table 5. Concordance rates and κ-coefficients by age of children

TST, Tuberculin skin test; QFT, QuantiFERON-TB® Gold test.
* P=0·972 in QFT and P=0·223 in TST positive rate for trend.
† P=0·007 with McNemar test for difference between QFT and TST positive rate.
Factors associated with positive test results
Univariate and multivariate analyses were performed to evaluate the covariates of children including sex, age, BCG scar, period between the diagnosis of the index case and the test, and smear positivity of index cases for positive TST and QFT. Smear positivity of index cases was the most important risk factor for positivity of both TST and QFT (Table 6). The smear-positive groups had significantly higher odds ratios of positive QFT compared with the smear-negative group in the multivariate analyses [i.e. 4·05 (95% CI 1·04–15·75) in smear-positive grade 1+, 4·09 (95% CI 1·00–16·66) in smear-positive grade 2+, and 9·72 (95% CI 2·28–41·46) in smear-positive grade 3+]. For the TST in the multivariate analyses, the odds ratios of a test result being positive to the smear-negative group were 1·50 (95% CI 0·56–4·04) in smear-positive grade 1+, 2·25 (95% CI 0·81–6·30) in smear-positive grade 2+, and 4·41 (95% CI 1·46–13·29) in smear-positive grade 3+.
Table 6. Odds ratios of positive-test results by covariate

TST, Tuberculin skin test; QFT, QuantiFERON-TB® Gold test; BCG, Bacille Calmette-Guérin.
* P<0·05, ** P<0·01.
To examine the effects of the age of children on the positive test results and simultaneously to assess the odds ratio of a given age between the two tests in terms of BCG vaccination at birth and environmental NTM infections occurring during growth, we compared the odds ratio in each age group with that in the 2 years age group, where the same positive rate of 0·17 was observed with both QFT and TST, and the concordance rate was highest at 0·95. The odds ratios for positive TST in the multivariate analysis were higher with 1·26 (95% CI 0·34–4·77) for the <1 year group, 1·02 (95% CI 0·33–3·14) for the 1 year age group, 3·91 (95% CI 1·31–11·72) for the 3 years age group, and 1·61 (95% CI 0·54–4·82) for the 4–5 years age group; however, these were not statistically significant except for the age group of three years. The odds ratio of positive QFT in the multivariate analysis was 2·09 for the 3 years age group, but was not statistically significant (95% CI 0·66–6·63). The presence of a BCG scar was not associated with positivity of either TST or QFT.
Time intervals of ⩾12 weeks between the diagnosis of the index case and the test seemed more likely to affect positive QFT results than those of 9–11 weeks, although the effect was not statistically significant in the multivariate analysis [odds ratios 2·49 (95% CI 0·62–9·98) for 12–15 weeks, and 3·23 (95% CI 0·80–12·96) for 16–20 weeks].
DISCUSSION
Several studies [Reference Mori5–Reference Ferrara11] have revealed that IFN-γ assays are highly specific and sensitive for detecting M. tuberculosis infection among adults and adolescents. Our concern with the QFT test is whether or not it will be useful and whether it can replace TST in detecting latent TB infection in younger children. Although some reports [Reference Dogra13–Reference Hill18] have demonstrated the usefulness of IFN-γ assays in children, few published studies have dealt with a large-scale comparison of QFT and TST in children aged ⩽5 years in developing countries.
The test results obtained with QFT in this study were fairly comparable with those with TST, with an overall concordance rate of 0·88 and a κ-coefficient of 0·63. A history of BCG vaccination did not significantly affect the TST or QFT results; however, the unavailability of accurate BCG histories may have masked potential associations to some extent. Previous reports [Reference Mori5, Reference Brock6, Reference Kang8] have stated that BCG vaccination causes false-positive results with TST among adults and therefore IFN-γ assays are more specific than TST. Reactivity to TST after BCG immunization varies with many factors (e.g. the age of the child at vaccination, the amount of time since vaccination, and the frequency of administration) [Reference Rieder23, Reference Wang24]. It has been reported that TST results are independent of previous BCG vaccination in developing countries where BCG is usually administered at birth or in infancy [Reference Lockman25, Reference Chadha, Jagannatha and Kumar26], whereas in low- or intermediate-burden countries, BCG adversely affects the specificity of TST [Reference Mori5, Reference Ewer7, Reference Mazurek10].
We observed higher odds ratios of positive TST in the <1 year and ⩾3 years age groups compared to the 2 years age group in the multivariate analysis, whereas the odds ratio of positive QFT exceeded one only for the 3 years age group. This result may be due to a lower sensitivity of QFT for detecting latent TB infection, although most published data do not support such lower sensitivity [Reference Rothel and Andersen27]. It is possible that in the <1 year age group, the BCG vaccination given a short period prior to the test affected TST, inflating the positive rate, although it did not affect QFT. This effect of BCG may have diminished after more than 1 year, as indicated in some studies [Reference Lockman25, Reference Chadha, Jagannatha and Kumar26]. Another possible explanation is false-positive TST tests caused by NTM infections in older age groups or in BCG-vaccinated individuals, with exposure to environmental mycobacteria having boosted the response originally induced by the vaccination. The national prevalence survey in Cambodia [28] revealed an increasing proportion of those having 5–14 mm induration in TST, which is considered to be mainly attributable to NTM infections [Reference Von Reyn29] among children aged 1–4 years compared with children aged 5–9 years. In the present study, the proportion of 5–14 mm induration in children aged ⩾3 years was 55% (45/82), significantly larger than the 38% (43/113) in children aged <3 years (χ2=5·43, P=0·020). Of course, this result could be at least partly attributed to the BCG vaccination effect, with or without boosting by NTM; however, this effect was not confirmed in the present study, due to the difficulty of ascertaining BCG history. The idea that the TST results were confounded by BCG vaccination and/or NTM infections may also be supported by the finding that the QFT-positive rate among weak TST-positive reactors with induration of 10–14 mm was lower than that among strong positive reactors with induration of ⩾15 mm, suggesting some false-positive reactions in the former group.
The interval between exposure to M. tuberculosis and detectable QFT reactivity (i.e. the window or incubation period) has not yet been established, although it is believed to be similar to that for TST (i.e. 8 weeks in practice) [12, 30]. In our study, time intervals of ⩾12 weeks between the diagnosis of the index case and the test were associated with positive QFT results, although they were not statistically significant by logistic regression analysis. This result may suggest that QFT takes longer than TST to convert to test-positive because the two tests probably do not reflect the same components of the cellular immune response [Reference Orme and Collins31]. Additional findings should be accumulated in the planned follow-up study. To statistically detect the difference between 9% and 17% observed in the present study with 95% confidence interval and 80% power, 301 subjects would need to be tested in each group.
Nine children had indeterminate QFT, indicated by a low response to the test's mitogen-positive control. This result suggests an impaired immune status and/or improper performance of the test [22]. It has been shown that indeterminate results are significantly overrepresented in patients with a negative TST and are more frequent in patients receiving immunosuppressive therapy [Reference Ferrara11]. In our study, eight of the nine children with indeterminate results had a negative TST (Fisher's exact P in comparison with the other 195 determinate cases=0·331). We did not test the subjects for HIV, and the lack of data on HIV status is one of the limitations of our study. However, it is roughly estimated that 3% of the children in this study may have been infected with HIV, considering that 10% of TB patients, male as well as female, are HIV positive in Cambodia [32] and that the risk of infection from mother to infant through vaginal delivery is estimated at 30%. It is therefore reasonable to assume that of a total of 217 children, nine (4·1%) may have had HIV. In addition, adequate blood specimens from 13 children (6·0%) were not drawn for mitogen-positive control in QFT and the test results were not obtained. This proportion can be lessened further for diseased children in hospital, because we did not force the nurses to draw blood specimens from healthy subjects.
Another concern is that we applied the same 0·35 IU/ml positive cut-off point to children as to adults, because a positive cut-off point for QFT has not been established specifically for children. In Japan, 0·1–0·35 IU/ml is defined as ‘intermediate’ or ‘doubtful,’ meaning that other factors relevant to the risk of infection should be taken into account in deciding the best action for the tested subjects [21]. This double-standard strategy is similar to the case of TST as adopted in the United States [33]. If we had used this criterion in our study series, another 19 (9·7%) of the 195 children with negative QFT would be judged as ‘intermediate’ or ‘doubtful.’ Since eight subjects (42%) had a positive TST, the concordance between the two tests would be increased. Further study is required to determine an appropriate cut-off point for young children.
Considerably low positive rates for the two tests were obtained from the 19 children diagnosed with active TB, probably due to over-diagnosis by our following the NTP guidelines, which originally targets symptomatic children seeking medical care and intends to avoid overlooking active TB among them. We did not assess their test results here, because our main objective was to determine the association between the two tests, not the validity of the guidelines.
We did not use PPD with the international standard of 5 TU of PPD-S or 2 TU of RT23, but with the equivalent of 2·5 TU of PPD-S, As shown in the results, we used a cut-off point of 10 mm for TST, which was obtained from the antimode of the bimodal distribution in the responses to the PPD injected. Thus, the effect of PPD products on the TST results is considered to be negligible. If we had used a cut-off point of 9 mm or 11 mm, the overall positive TST rate would have been 27% or 22%, respectively, and the difference is small in comparison with our finding of 24%.
For older children who may have a greater TST response [28], QFT is likely to have the advantage even in developing countries such as Cambodia or India [Reference Dogra13], of avoiding the effects of NTM infection. Although logistical as well as economic problems are involved in using QFT, it will become a strong technology for epidemiological and immunological studies of TB control, enabling us to estimate the annual risk of infection of M. tuberculosis more reliably and to make clinical diagnoses of paediatric TB in the developing world as well as in industrialized countries.
In summary, QFT can potentially replace TST in detecting latent TB infection in childhood contacts aged ⩽5 years, especially in those who may have a false-positive TST due to BCG vaccination or NTM infection.
ACKNOWLEDGEMENTS
This study was partly supported by Japan International Cooperation Agency. The authors acknowledge the following people for technical assistance and input in this study: Drs Pichenda Koeut, Sokonth Keo, Chhom Sayoeun Prum, Satha Peou, Sivanna Tieng, and Kim Eam Khun (National Center for TB and Leprosy Control, Cambodia); Drs Rin Oung, Vuthy Un, and Kamhouy Chy (National Pediatric Hospital, Cambodia); and Drs Thai Veng, Chharan Thong, and Thorn Nim (Medical Officer of Health Department, Cambodia). The authors also thank Professor Nobufumi Yasuda (Kochi University, Japan) and Dr Norio Yamada (Research Institute of Tuberculosis, Japan Anti-Tuberculosis Association, Japan) for advice on statistical analysis of the data.
DECLARATION OF INTEREST
None.









