INTRODUCTION
There is abundant evidence that early childhood adversity influences adult health outcomes, with factors such as low childhood socioeconomic status (SES), poverty, and poor or overcrowded housing being associated with increased risk of cardiovascular, respiratory and psychiatric illness [Reference Kuh and Ben-Shlomo1–Reference Poulton3]. These effects may be mediated in part through stress processes, since childhood abuse, family discord and negative life events increase risk for later ill health [Reference Dong4–Reference Evans and Kim6], and tend to cluster in children of lower SES [Reference Taylor7]. Childhood stressors also impact on biological processes relevant to adult disease risk [Reference Taylor8, Reference Danese9]. Extensive research both in animals and humans has documented the impact of early psychosocial adversity on neuroendocrine function in adult life, with effects on cortisol regulation and stress reactivity [Reference Elzinga10, Reference Heim11]. Childhood SES is associated with aberrant profiles of cortisol over the day independently of adult SES [Reference Li12]. Stress-induced neuroendocrine dysregulation has been proposed as a key process linking early life experience with adult risk of physical disease and psychopathology [Reference Gunnar and Quevedo13–Reference Belmaker and Agam15]. Cortisol has a pronounced diurnal rhythm, with high levels early in the morning, declining throughout the day to a nadir in the late evening and early hours of sleep [Reference Van Cauter16]. The full diurnal profile requires repeated blood sampling, usually in clinical or laboratory settings, but the introduction of salivary cortisol measurements has permitted the profile over waking hours to be assessed in large samples in everyday life [Reference Kirschbaum, Hellhammer and Fink17]. Different aspects of the diurnal profile appear to be associated with SES and other psychosocial factors, notably the cortisol awakening response (CAR; the rise over the first 30–45 min after waking), the overall cortisol output over the day, and the slope of cortisol decline over the day [Reference Kirschbaum, Hellhammer and Fink17, Reference Chida and Steptoe18].
Another pathway through which early life adversity may influence adult disease risk is exposure to common pathogens. Thus seropositivity for cytomegalovirus (CMV), Chlamydia pneumoniae (C. pneumoniae), herpes simplex virus 1 (HSV-1), and Helicobacter pylori is greater in individuals of low SES defined by education, poverty or occupational status, and with household crowding [Reference Dowd19–Reference Zajacova25]. These pathogens are typically acquired in childhood [Reference Dowd26], when they may or may not be symptomatic [Reference Smith and Robinson27–Reference Malaty29]. Reactivation of specific pathogens such as CMV and HSV-1 may be relevant to disease risk and cognitive decline in older age [Reference Aiello30, Reference Sorlie31]. In addition, cumulative pathogen burden, defined by positive serostatus for a range of pathogens, has been associated with cardiovascular risk factors and with coronary heart disease (CHD) in case-control and longitudinal cohort studies [Reference Espinola-Klein32–Reference Kiechl35].
The relationship between pathogen burden and cortisol profile in adults has not been extensively investigated. Animal studies have demonstrated that early-life exposure to endotoxin alters later-life hypothalamic–pituitary–adrenocortical (HPA) function, with raised corticosterone output over the day [Reference Shanks36]. Glucocorticoids are known to enhance the growth of C. pneumoniae in cell cultures [Reference Komura37] and the replication of human CMV in embryonic cells [Reference Tanaka38]. Additionally, early childhood exposure to upper respiratory infection was negatively correlated with cortisol sampled in the clinic and in the late evening in the Barry Caerphilly Growth cohort [Reference Vedhara39]. In this study, we hypothesized that cumulative pathogen burden, defined by positive serostatus for C. pneumoniae, CMV and HSV-1, influences cortisol regulation in adult life. We measured salivary cortisol over the day and evening, specifically focusing on total cortisol output, the CAR, and the slope of cortisol decline over the day. A blunted CAR has been associated with cardiovascular risk factors [Reference Wirtz40], while a flattened cortisol profile over the day is related to subclinical coronary artery disease [Reference Matthews41] and abdominal adiposity [Reference Rosmond42]. Other factors such as age, gender, low SES, body mass, smoking, depression, health status, and time of waking have been related to cortisol profiles over the day, and were therefore taken into account in the analyses.
METHODS
Participants
Data were analysed from 361 members (237 men, 124 women) aged 51–72 years from the Whitehall II epidemiological cohort [Reference Marmot43]. Data were collected from consecutive attendees at a medical screening session where we completed assessments of serostatus for C. pneumoniae, CMV, and HSV-1, as detailed elsewhere [Reference Steptoe20]. All participants were in good health, with no indications of current infection. In total, 378 participants carried out salivary cortisol assessments over the day and evening, of whom 17 were excluded because they were taking either oral or inhaled corticosteroid medication. The individuals taking part in this substudy did not differ from the remaining healthy participants in the medical screening in terms of age, gender and SES. None of the participants were shift-workers. The study was approved by the University College London/University College London Hospitals Medical Ethics Committee, and all participants gave signed consent.
Measures
Participants underwent a physical examination during which fasting blood samples were obtained. Height and weight were measured, and body mass index (BMI) was computed. Current or most recent occupational grade in the civil service was used to index SES, and participants were divided into higher, intermediate and lower grade groups. Self-rated health was measured with a single item (‘In general, would you say your health is: excellent, very good, good, fair, or poor’) that has consistently been shown to predict adverse health outcomes [Reference Idler and Benyamini44]. Depressed mood was measured using the Center for Epidemiologic Studies Depression (CES-D) scale. Current medication was also assessed.
Salivary sampling with salivettes (Sarstedt, UK) was explained and practised during the health screening. Participants were instructed to collect saliva immediately after waking, 30 min later, and then at 2½ h, 8 h and 12 h after waking, with a final sample being obtained just before bedtime. They were instructed to record the time of sample collection, take samples as soon as they woke, avoid caffeine and acidic drinks in the first 30 min, and not brush teeth or eat or drink anything for 15 min before a sample collection. Time of waking was recorded along with the actual times the saliva samples were collected. Participants stored salivettes in domestic refrigerators before posting them back to the laboratory. Saliva sampling took place within 7–10 days of the collection of blood samples used for the analysis of seropositivity.
Laboratory methods
Serostatus for C. pneumoniae, CMV, and HSV-1 was assayed at Umeå University using methods described previously [Reference Steptoe45]. Briefly, C. pneumoniae IgG was measured with the Chlamydia microtitre indirect immunofluorescence test (Focus Technologies, USA) using a serum dilution of 1/16. Each serum was tested against antigen from C. pneumoniae and a control antigen. Two control sera were used, one seropositive and one seronegative. The results were read manually by fluorescent microscopy by two independent investigators and sera with a specific apple green fluorescence were considered seropositive. Sera reacting with the control antigen were not possible to interpret and therefore excluded from the analyses. All procedures and interpretation were performed according to the manufacturer's instructions. The antigen for the CMV IgG ELISA was derived from CMV-strain Ad169 cultured in human fibroblasts. The antibody activity was expressed in arbitrary units (AU); a value of ⩾10 AU was considered positive for presence of IgG and <5 AU as negative. A grey zone area was defined in the range between 5–9 AU, but participants who fell into this range were classified conservatively as seronegative. The antigen for the HSV-1 IgG ELISA was a local HSV-1 strain cultured in GMK-cells. Both tests followed the protocols and materials used for clinical tests accredited by SWEDAC (Swedish Board for Accreditation and Confirmatory Assessment), and external international quality assurance programmes from Labquality and the UK National External Quality Assessment Service validate the tests several times a year. The sensitivity of detecting these latent viral infections is estimated to be almost 100%. The specificity is also very high since each serum sample is controlled for unspecific reactivity by a control antigen. The high and low reacting control sera used have been tested at two independent accredited laboratories according to standard accreditation regulations.
Salivary samples were sent to the Technical University Dresden for the analysis of cortisol. The salivettes were centrifuged at 3000 rpm for 5 min, resulting in a clear supernatant of low viscosity. Salivary cortisol levels were measured using a commercial immunoassay with chemiluminescence detection (CLIA; IBL-Hamburg, Germany). The lower limit of the assay was 0·44 nmol/l, and intra- and inter-assay coefficients of variance were <8%.
Statistical analysis
Delays between waking in the morning and taking the ‘waking’ cortisol sample can lead to inaccurate waking values and a diminished CAR [Reference Dockray46]. We excluded 44 individuals who reported a delay of more than 15 min between waking and taking sample 1, leaving 317 in the analysis. There were no differences in age, gender, seropositivity, self-rated health or other factors between those who were included and excluded from the final analysis. Cumulative pathogen burden was defined as the number of pathogens (C. pneumoniae, CMV, HSV-1) for which the individual was seropositive, so could range from 0 to 3. The associations between pathogen burden and age, gender, SES (grade of employment), BMI, smoking status, self-rated health, depression and time of waking were assessed using analysis of variance for continuous measures and χ2 tests and logistic regression for categorical variables. The most common medications were related to cardiovascular health, including antihypertensives and statins. The use of cardiovascular medication was therefore included as an additional covariate. Total cortisol output over the day was computed using area under the curve methods involving all six samples [Reference Pruessner47]. The slope or rate of decline in cortisol over the day was computed by calculating regression slopes across all data-points except the 30-min post-waking sample (sample 2), and was expressed as nmol/l per h. The CAR was assessed as the difference between samples 1 and 2. The cortisol output, cortisol slope over the day and CAR were normally distributed, while levels on waking and bedtime were skewed, and therefore log-transformed before analysis. The relationship of cortisol with burden of infection was assessed with analysis of covariance, adjusting for age, gender, SES, BMI, smoking status, self-rated health, depression and time of waking. Results are presented as means±standard deviations.
RESULTS
The 317 participants in the analysis included 205 men and 112 women aged 61·0±5·7 years. Serological status for the three pathogens is summarized in Table 1. The pathogen burden was 0 in 9·8%, 1 in 27·8%, 2 in 39·1% and 3 in 23·3%, with a mean of 1·76±0·92. Pathogen burden did not differ between men and women, but was positively related to age (P=0·004). As previously described, participants of lower SES tended to have a greater pathogen burden (P=0·016) [Reference Steptoe20]. Pathogen burden was positively associated with BMI, but was not related to smoking, self-rated health, cardiovascular medication, depressed mood or time of waking in the morning (Table 2).
Table 1. Pathogen burden

Table 2. Participant characteristics associated with pathogen burden
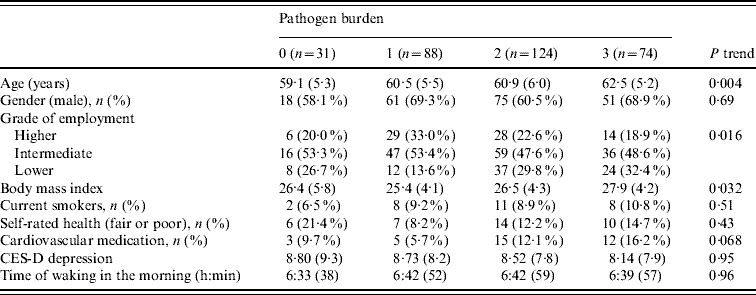
Results expressed as means (standard deviation) and n (percent).
Figure 1 illustrates the profile of salivary cortisol over the study. Participants woke at 6:41 on average, and took the 30-min post-waking sample at 7:12±56 min. Subsequent measures were taken at the expected intervals. Cortisol increased by 6·88±10·4 nmol/l between waking and 30 min later, falling over the remainder of the day. Cortisol output over the day averaged 7·87±2·87 nmol/l, while the rate of cortisol decline over the day averaged 1·204±0·89 nmol/l per h.
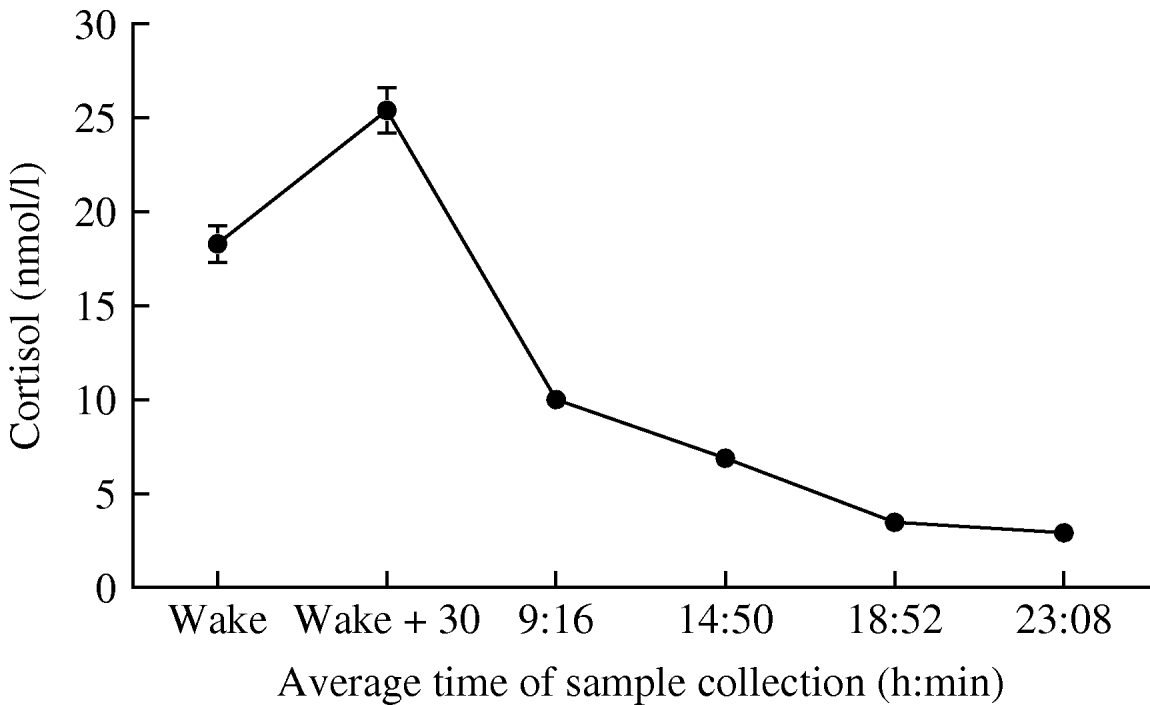
Fig. 1. Mean salivary cortisol at the six assessment points over the day. Error bars are standard error of the mean (s.e.m.).
There was no relationship between pathogen burden and total cortisol output over the day (Table 3). This was also true when the three individual pathogens were tested. However, there was a significant linear association between pathogen burden and cortisol decline over the day after adjustment for age, gender, grade of employment, BMI, smoking status, self-rated health, cardiovascular medication, depression and time of waking (P=0·010). As can be seen in Figure 2, the rate of cortisol decrease over the day was greatest in participants with zero pathogen burden, and least in those with the highest pathogen burden. This indicates that the cortisol rhythm over the day was flatter in participants with greater pathogen burden, independently of covariates associated with pathogen exposure and cortisol output. When we analysed relationships with individual pathogens, a flatter cortisol slope was associated with seropositivity for CMV (P<0·001), but not the other pathogens in isolation. Results were unchanged when waist/hip ratio was included as an additional covariate.
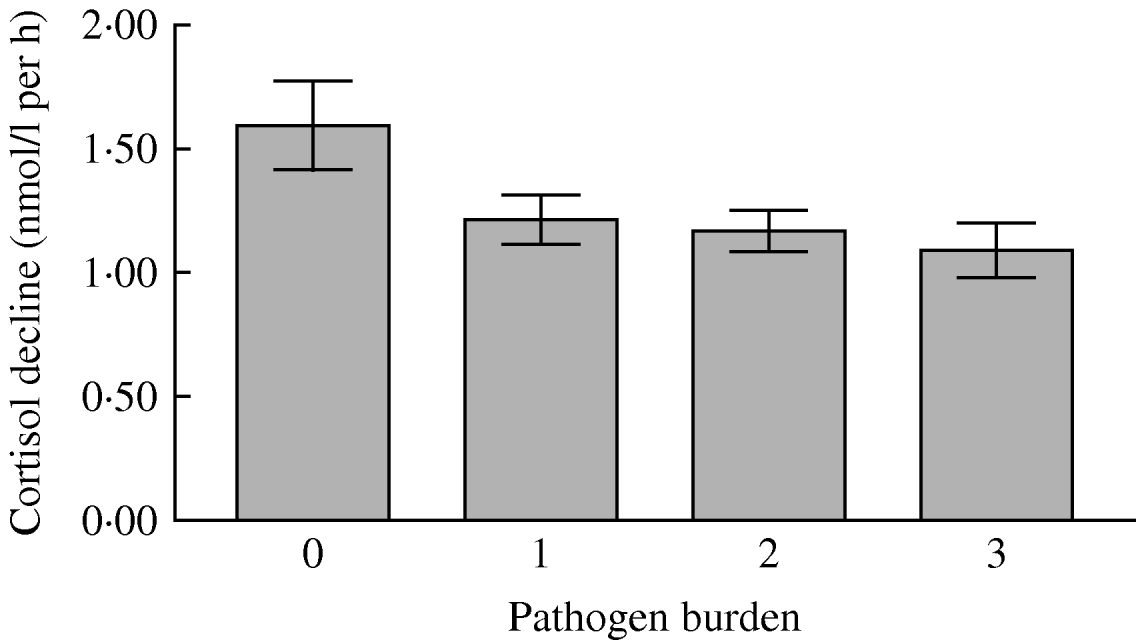
Fig. 2. Mean rate of salivary cortisol decrease over the day in participants with infectious burden scores of 0, 1, 2 or 3. The difference was significant after adjustment for age, gender, grade of employment, body mass index, smoking status, self-rated health, cardiovascular medication, depressive symptoms and time of waking in the morning. Error bars are s.e.m.
Table 3. Salivary cortisol in relation to pathogen burden
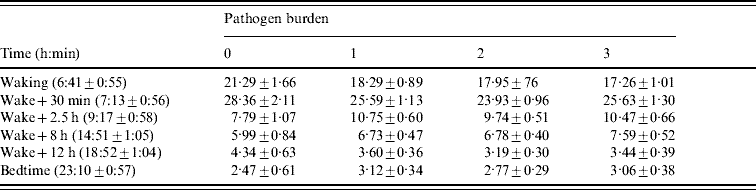
The times of sampling are presented as means±standard deviation. Cortisol results are presented expressed as means±standard error, adjusted for age, gender, grade of employment, body mass index, smoking status, self-rated health, cardiovascular medication, depression and time of waking.
The flatter cortisol decline over the day in participants with a greater pathogen burden was driven primarily by differences in cortisol levels on waking. Separate analysis of the waking values showed a significant association with pathogen burden after adjustment for age, gender, SES, BMI, smoking status, self-rated health, cardiovascular medication, depression and time of waking (P=0·031). Cortisol levels on waking were higher in participants with zero pathogen burden scores. By contrast, pathogen burden was not related to cortisol at bedtime (P=0·63), although values were lowest in participants with zero pathogen burden.
There was no association between pathogen burden and CAR (P=0·58). The CAR averaged 6·76±10·1, 7·34±10·3, 6·18±10·1 and 8·58±11·0 nmol/l in participants with burden scores of 0, 1, 2 and 3 respectively, after adjustment for covariates.
DISCUSSION
Cumulative pathogen burden has been postulated to be a risk factor for CHD, contributing to atherosclerosis [Reference Steptoe20, Reference Espinola-Klein32, Reference Zhu33]. Exposure to pathogens is greater in lower SES groups and in individuals who experience poverty and adversity in early life. Since early stress and adversity are related to HPA regulation, we reasoned that older adults with greater pathogen burden scores might show disturbances of cortisol output. It should be emphasized that these analyses of associations between cortisol output over the day and cumulative pathogen burden are preliminary, since only three pathogens contributed to the cumulative burden index, and salivary cortisol was measured over only a single day. Nevertheless, we found that pathogen burden was associated with a flatter cortisol rhythm over the day, independently of factors that potentially influence cortisol including age, gender, current SES, smoking, BMI, depression, self-rated health, and time of waking in the morning. The flatter cortisol slope was driven primarily by lower cortisol values on waking in participants with greater pathogen burden, rather than raised evening values. Flatter cortisol slopes appear to be associated with increased health risk, and have been related in other studies to coronary disease and abdominal adiposity [Reference Matthews41, Reference Rosmond42]. A longitudinal study of patients with breast cancer found that flatter cortisol slopes predicted early mortality, independently of covariates [Reference Sephton48]. Additionally, flatter cortisol slopes are associated with some aspects of psychological stress and coping; e.g. men and women with marital problems have flatter cortisol slopes [Reference Adam and Gunnar49, Reference Barnett50], while individuals who utilize adaptive coping styles have steeper slopes [Reference O'Donnell51]. By contrast, there was no association with peak cortisol, suggesting that the different components of the cortisol diurnal rhythm have varying associations with health-related biological processes. Previous studies have shown limited associations between cortisol slopes, peak levels, and average output [Reference Stone52].
The mechanisms underlying the association between pathogen burden and cortisol rhythm are not certain. The effect appeared to be driven primarily by CMV seropositivity, although the significant trend across pathogen burden categories indicates that C. pneumoniae and HSV-1 also contributed. There are four broad possibilities. First, there could be a direct effect of pathogens on HPA function. Infection with these common pathogens is likely to have taken place early in life [Reference Dowd19, Reference Dowd26, Reference Xu53]. CMV is a potent immunomodulator [Reference Mocarski54], and is believed to contribute to T-cell immunosenescence [Reference Koch55]. Viral infections are associated with concomitant activation of the HPA, and cytokines released in response to viral infection induce corticotrophin-releasing factor (CRF), have direct effects on adrenocorticotropic hormone (ACTH) release from the anterior pituitary, and promote glucocorticoid secretion from the adrenal glands [Reference Silverman56]. Levels of mRNA in the paraventricular nucleus of the hypothalamus and the peptides corticotrophin-releasing hormone and arginine vasopressin in the median eminence are raised by endotoxin treatment in early life, and may lead to resetting of adult HPA function [Reference Shanks57]. HSV-1 may cause down-regulation of glucocorticoid receptors in the hippocampus, thereby augmenting HPA activation [Reference Bener58]. These responses could lead to chronic HPA dysregulation expressed as a flattened diurnal cortisol profile, although direct mechanistic evidence is currently lacking.
A second possibility is that disturbed cortisol profiles may have preceded infection and increased susceptibility. Even though cortisol was assessed in middle-age whereas infection was probably acquired early in life, the temporal relationship between infection and cortisol cannot be determined in this cross-sectional design. However, administration of glucocorticoids to human cell cultures enhances the growth and replication of CMV and C. pneumoniae [Reference Komura37, Reference Tanaka38]. Third, exposure to infection in childhood and psychosocial adversity may coexist. Acquisition of a variety of pathogens early in life is greater in children from low SES backgrounds [Reference Dowd19, Reference Xu22, Reference Dowd59], and living in poorer, crowded residences may increase risk of family conflict and maltreatment [Reference Poulton3]. These factors can in turn stimulate HPA dysregulation that is manifested in adult life [Reference Gunnar and Quevedo13, Reference Luecken and Lemery14]. If this is the case, then infectious burden could be a marker of stressful experiences in early life that lead to HPA dysfunction, and have no direct role. Interestingly, other population studies have shown differential associations between childhood SES and different measures of cortisol rhythm in adult life, consistent with the current findings [Reference Li12].
A fourth possibility is that the association is due to stress-related increases in antibody titres for latent infections. There is a growing literature relating antibody titres with psychological stress [Reference Glaser60, Reference Phillips61], and evidence that cortisol is involved in the up-regulation of viral replication [Reference Glaser62–Reference Sainz64]. It has recently been shown that HSV-1 antibody levels are heightened in adolescents who experienced severe stress or abuse as children [Reference Shirtcliff65]. It is theoretically possible that our results were related to reactivation of pathogens, increasing the likelihood that antibody levels exceed the threshold for seropositivity tests. However, this is unlikely for two reasons. First, the detection thresholds in the tests for serostatus were set low. Second, we found no association between cortisol and the levels of CMV or HSV-1 antibodies in analyses limited to individuals who had positive serostatus (results not presented).
This study has several limitations. The sample was weighted towards men, as is also the case in the larger Whitehall study from which participants were drawn [Reference Marmot43]. We have no evidence about when seropositivity for these infectious agents was acquired. We only tested three pathogens, and other organisms are relevant to pathologies such as CHD, notably periodontal infectious agents [Reference Spahr66]. Multiple days of cortisol monitoring would have been desirable, since measures from a single day may not be representative [Reference Hellhammer67]. Despite adjusting statistically for the principle influences on cortisol regulation, unmeasured factors correlated with infection exposure may have affected cortisol output. We were not able to measure ACTH in our study, and this would have been valuable for the understanding of cortisol dynamics. Nor was it possible to conclude whether overall exposure to cortisol throughout the complete diurnal cycle differed between pathogen burden groups, since values were not recorded in the night. Nevertheless, this study on a large well characterized population suggests that cumulative pathogen burden is positively related to cortisol dysregulation. Since early life psychosocial adversity frequently coincides with pathogen exposure, this association may be relevant to the processes through which life experience affects HPA function in health and disease.
ACKNOWLEDGEMENTS
This research was supported by the British Heart Foundation (grant PG/03/029). Västerbotten County Council provided financial support for Å.G. We thank the men and women in the Whitehall II cohort and the Whitehall study team for their participation.
DECLARATION OF INTEREST
None.







