INTRODUCTION
Noroviruses are the leading known cause of foodborne disease outbreaks in the USA [1]. Noroviruses may also be spread through direct person-to-person contact or environmental contamination. Consequently, secondary transmission often follows a point-source food exposure [Reference Becker2]. Shellfish, primarily oysters, are commonly implicated vehicles of foodborne norovirus outbreaks, particularly consumed raw or inadequately cooked [3]. While most foodborne norovirus outbreaks are believed to result from contamination by an ill food handler at the point of service, oyster-associated norovirus outbreaks often result from contamination at the source due to faecally contaminated growing waters. Oyster beds may become contaminated due to land-based sewage outflow or sewage disposal from oyster harvesters [Reference Le Guyader4, Reference Kohn, Thomas and Ando5]. Oysters are capable of significant bioaccumulation of virus in the flesh and gut to concentrations up to 99 times greater than that of the surrounding waters during the autumn/winter season [Reference Burkhardt and Calci6] and remain infectious even after depuration [Reference McLeod7].
During December 2009, over 200 calls from individuals reporting gastrointestinal symptoms after dining at a restaurant in North Carolina prompted a multi-agency investigation. The objectives of this investigation were to characterize the extent of the outbreak, identify the cause and source of illness, and recommend appropriate control measures to prevent further spread.
METHODS
Case-control study
Initially, information about the outbreak was gathered through telephone interviews from restaurant patron complaints to state and local authorities. A case-control study was then undertaken to determine risk factors associated with illness after eating at the restaurant. A case was defined as any person who ate at the restaurant during 10–22 December 2009 and developed vomiting or diarrhoea (⩾3 loose stools in 24 h) or both ⩽72 h after eating at the restaurant. Cases were selected from the complaint records of calls to the local health authority from ill restaurant patrons and from the restaurant, and 177 individuals met the case definition. Due to resource limitations, 51 records were randomly selected for study inclusion using random number selection in Excel (Microsoft, USA) software. Controls were defined as well persons who dined at the restaurant during 10–22 December 2009 and who did not have diarrhoea or vomiting within 3 days of eating there. Eighty controls were selected from among well dining companions of cases ascertained during case interviews, names on credit card receipts and reservations acquired from the restaurant and direct reports to the study team about well persons eating at the restaurant.
A brief questionnaire including food items reported consumed during initial complaint interviews was administered to cases and controls by telephone interview. Permission to interview was obtained verbally from each person before the interview was conducted. Data were analysed by univariate and stratified analysis to control for potential confounding exposures using Epi-Info software version 3.3.2 (CDC, USA).
Household transmission study
To further characterize the extent of the outbreak and quantify the secondary attack rate, a household transmission study was also conducted. Primary cases and corresponding contact information were identified from restaurant complaints received by state and local public health authorities and interviewed by phone using a brief, scripted questionnaire. Only primary cases not randomly selected to participate in the case-control study were contacted. Information on all members of the household was collected, including age, sex, dining history at the restaurant, gastrointestinal illness, and specific symptoms. An exposed household member was defined as someone who did not dine at the restaurant but slept in the household at least half of the time during the 2 weeks following the primary case's visit to the restaurant. A secondary household case was defined as an exposed household member who developed vomiting or diarrhoea within 14 days after illness onset of the primary case. Households were excluded from analysis if all household members dined at the restaurant, there was only one person in the household, no one in the household met the primary case definition, or a primary case in the household dined at the restaurant on multiple occasions. Households were recorded as non-responders after three unsuccessful attempts by phone contact. Secondary attack rates were calculated at both the household and individual levels. Potential risk factors for infectivity were assessed by comparing clinical and demographic characteristics of the primary household case in households with and without secondary household cases. Similarly, potential risk factors for susceptibility (e.g. age of secondary cases) were assessed by comparison of exposed household members that became secondary cases with those that remained well. Univariate analyses were performed by Mantel–Haenszel or Fisher's exact χ2 test using Epi-Info software version 3.4.3 (CDC, USA).
Laboratory analysis
Stool specimens
A total of six stool specimens were collected from primary cases during the week of 21–28 December. The collection dates ranged from 3 to 10 days after illness onset. Additionally, eight stool specimens were collected from eight asymptomatic restaurant employees during the week of 28 December.
Five of six specimens from primary cases and all eight specimens from the food handlers were analysed at the NC State Laboratory of Public Health (SLPH) using TaqMan real-time reverse transcription–polymerase chain reaction (rRT–PCR) methodology [Reference Trujillo8]. One specimen from a primary case could not be analysed because of insufficient volume. Four of the five stool specimens from primary cases and all eight stool specimens from the food handlers were sent to CDC laboratories for confirmation and genetic sequencing using conventional RT–PCR [Reference Vinje, Hamidjaja and Sobsey9].
Oyster samples
At least one dozen oysters from each of four different lots were sampled and analysed for detection of norovirus RNA by the Environmental Virology and Microbiology Laboratory, University of North Carolina, Gillings School of Global Public Health. Two of the lots tested were from Louisiana harvest area 1 with harvest dates of 14 and 19 December 2009; the other two lots were from North Carolina harvest area G3 with harvest dates of 11 and 18 December 2009. One hundred grams of homogenized tissue from each of the four oyster lots were analysed [Reference Mullendore, Sobsey and Shieh10]. Conventional one-step RT–PCR and nested RT–PCR procedures were used for the detection of norovirus RNA using commercially available kits (Qiagen, USA). The primers used for the one-step RT–PCR reaction were MJV 12/13 and Reg A [Reference Vinje, Hamidjaja and Sobsey9] and for the nested PCR were Reg A and MP 290 [Reference Maloney11].
Environmental investigation
On 3 December 2009, site visits and a full restaurant inspection were initiated. Restaurant management was interviewed regarding staff duties and illness in restaurant workers. Given numerous reports of oyster consumption from ill restaurant patrons, further information was collected about the source and food-handling practices of the oyster products served at the restaurant.
RESULTS
Case-control study
Of the ~341 complaint records received from 2 to 23 December 2009, 177 meet the case definition (Fig. 1). Reported symptoms among these cases included diarrhoea (92%), vomiting (85%), nausea (31%), stomach cramps (23%), fever (19%), chills (14%), muscle aches (11%), and sweating (2·0%). The medium duration of illness was 24 h and medium incubation period was 25 h.
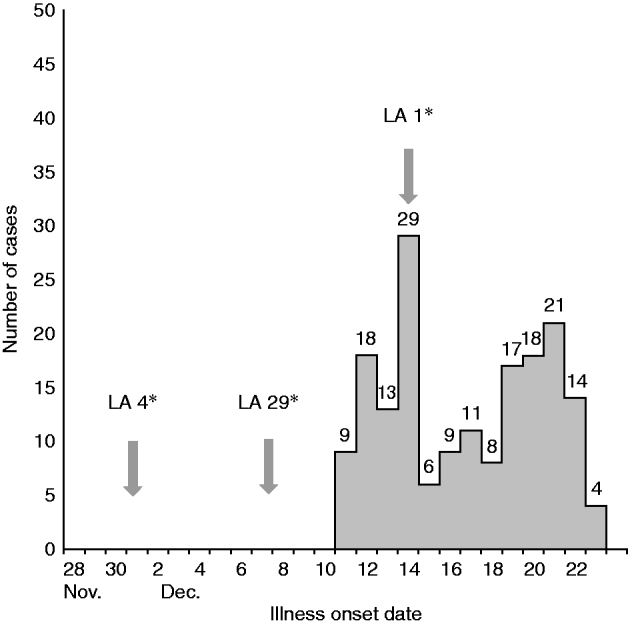
Fig. 1. Cases of gastroenteritis after eating at a restaurant by illness onset date (n=177). (For definition of a case see Methods section.) * Oyster delivery dates to restaurant from Louisiana (LA) harvest areas.
Compared with controls, cases were 13 times [95% confidence interval (CI) 4·3–39] more likely to have eaten any oysters and 12 times (95% CI 4·8–28) more likely to have specifically eaten steamed oysters (Table 1). Most (92·2%) of the cases ate any oysters and 82·4% ate steamed oysters. Cases were also more likely to have eaten cocktail sauce, horseradish, hot sauce, butter sauce, coleslaw, crackers and hushpuppies (deep-fried dumplings) compared to controls; however, through stratified analysis controlling for steamed oysters, the adjusted odds ratios for all of these foods (except horseradish) were not significant. Conversely, consumption of steamed oysters remained significant after individually controlling for coleslaw, cocktail sauce, and horseradish, with adjusted odds ratios of 9·2 (95% CI 3·6–23), 8·3 (95% CI 3·3–21), and 7·1 (95% CI 2·9–18), respectively (Table 2). Apart from steamed oysters, no other oyster dishes were significantly associated with illness.
Table 1. Univariate analysis for foods consumed in the case-control study
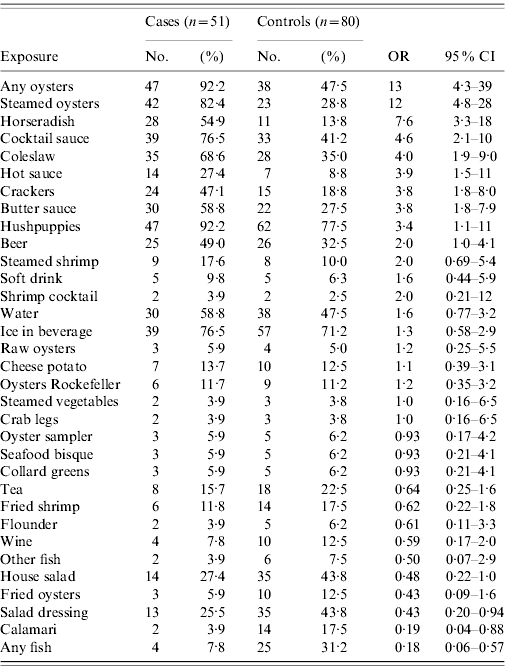
OR, Odds ratio; CI, confidence interval.
Table 2. Stratified analysis for foods eaten in the case-control study
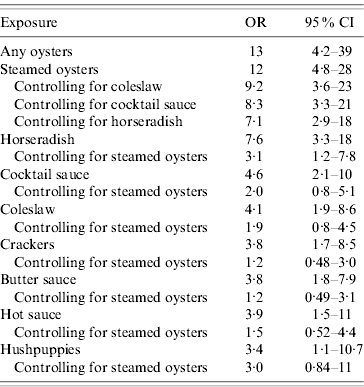
OR, Odds ratio; CI, confidence interval.
Household transmission study
Forty-one households were included in the secondary transmission analysis, comprising a total of 126 household members. Median household size was three persons, with a range of 2–8 persons per household. Among these individuals, 56 (44%) had dined at the restaurant, including 48 (38%) that were classified as primary cases, while 70 (56%) had not dined at the restaurant and were considered exposed to a primary case. A total of 10 (14%) secondary cases were reported among the 70 exposed household members, including four children and six adults. Symptoms reported in these 10 secondary cases included vomiting (80%), diarrhoea (40%), nausea (60%), fever (50%), abdominal cramps (40%), and headache (20%). By extrapolating the ratio of primary to secondary cases identified in the household transmission study (4·8:1) to the overall reported number of cases (177), we estimate at least 37 secondary household cases were associated with this outbreak. The median time between illness onset of the first primary case in the household and illness onset of secondary cases was 3 days (range 1–11 days). Overall, at least one secondary case was reported in eight (20%) of the 41 households included. Due to power limitations, no statistically significant risk factors for either infectivity or susceptibility were identified. However, a primary case with multiple vomiting episodes or multiple primary cases with vomiting were more frequent in households with secondary transmission (100% and 20%, respectively) than in those without (76% and 8%, respectively).
Laboratory analysis
Norovirus RNA was detected in three of five stool specimens from primary cases by the SLPH and subsequently confirmed by CDC in two of four specimens submitted. Norovirus RNA was not detected in any of the eight stool specimens from the food handlers or the oyster samples. Sequence analysis of the RT–PCR products from two positive stool specimens tested at CDC identified identical genogroup II genotype 12 (GII.12) sequences.
Environmental investigation
Oysters were steamed rare (2 min), medium (4 min) or well done (6 min) depending on the customer's request. Final cooking temperatures of 21–30°C for rare, 46–58°C for medium and 61–74°C for well done were measured using a digital thermometer by a North Carolina Registered Environmental Health Specialist (REHS) from the local health authority during the environmental investigation. Immediately after steaming, oysters were opened and served to the customer only at the oyster bar. Steamed oysters were served with coleslaw, cocktail sauce, drawn butter and sometimes horseradish. Kitchen staff prepared the coleslaw, and reportedly there was some mixing of the slaw with bare hands. Most of the workers' gloves and knives were only washed at the handwash sink and were not routinely sanitized. Quaternary ammonium sanitizer was used for disinfecting surfaces, utensils and equipment in the restaurant. No employees had reported recent illness during the outbreak period.
At the time of the investigation, raw oysters were received from two suppliers (A and B).
Supplier A provided oysters exclusively from Louisiana (LA) and Supplier B provided oysters from Mississippi, Texas and North Carolina harvest areas. Only supplier A oysters were continuously used for all steamed oysters served during the outbreak while supplier B oysters were used for raw and baked menu items. A review of the restaurant menu items that were implicated in the case-control study and the source of oysters used to make those items indicated that no food items made with oysters from supplier B were associated with illness. All of the oysters from supplier A were from Louisiana with harvest dates ranging from 15 November 2009 to 19 December 2009. Notably, the shipment from LA area 29 and harvest date 6 December 2009 was delivered on 8 December 2009, 3 days before the outbreak started. Oysters from LA area 1, harvest date 14 December 2009 was delivered on 15 December 2009. Both of these lots were served during the outbreak (Fig. 1). Oysters from LA area 1 were delivered at the peak of the outbreak and therefore could not have initiated the outbreak. However, based on interviews with oyster growers and regulators, the normally expected duration of harvested oysters remaining in the marketplace until consumption is about 2 weeks. Illness onset dates in this outbreak occurred from 11 to 23 December inclusive and align with the time of delivery to expected consumption of LA area 29 oysters at the restaurant. Therefore consumption of undercooked oysters from LA area 29 was a risk factor for illness in this outbreak. Following declaration by the North Carolina Division of Public Health of an epidemiological link between consumption of oysters and the outbreak, the Louisiana Department of Health and Hospitals closed LA 29 to harvest and recalled all oysters harvested from that area during 6–27 December.
DISCUSSION
We concluded this outbreak of gastroenteritis was caused by consumption of inadequately cooked oysters contaminated with norovirus and further amplified by secondary household transmission. Cases in the outbreak began within 3 days of oysters being delivered to the restaurant from LA harvest area 29, which is consistent with the incubation period for norovirus gastroenteritis [Reference Patel12] and continued during the time these oysters were served at the restaurant. Illness among food handlers was not reported and their stool specimens tested negative for norovirus. On 21 December, the restaurant voluntarily discontinued use of the remaining oysters from the LA harvest areas and no cases were reported after 23 December. These collective findings suggest that the oysters from LA harvest area 29 were probably contaminated with norovirus prior to arrival at the restaurant, either at the source or during distribution.
This study demonstrated that at least 20% of household members interviewed reported secondary cases representing a 14% secondary attack rate, consistent with previous reports of secondary household transmission following point-source norovirus outbreaks [Reference Marks13–Reference Baron17]. Based on these findings, we estimated that for every five primary cases identified, at least one secondary household case resulted. This highlights how exposure to a contaminated food vehicle, which may be widely distributed, can seed household and community transmission.
The following study limitations are noted. Given the highly infectious nature of norovirus and multiple modes of transmission through which it can be spread, the magnitude and extent of this outbreak is probably underestimated. Contributing factors for this underestimate include: the total number of patrons eating at the restaurant during the outbreak was unknown, the majority of cases were recognized only after a media release about the outbreak and the number of households in the household transmission study was low. The source of norovirus contamination was unknown and it was not possible to detect norovirus in oysters from LA area 29, harvest date 6 December 2009 because they were not available for testing. Oyster-associated norovirus outbreaks often involve multiple genotypes due to gross contamination. Sequence analysis of two positive specimens yielded the same GII.12 strain; however, it is unknown if additional norovirus strains could have been identified had more positive stool samples been available for sequencing.
A series of intervention measures were implemented at the restaurant during the outbreak to reduce the potential for additional illness including the following: cooking all shellfish products to an internal temperature of ⩾63°C for ⩾15 s, no bare hand contact with ready-to-eat foods, converting to chlorine-based sanitizer for use in the establishment, implementing a cleaning and sanitizing procedure for shucker gloves and knives, cleaning and sanitizing all ice-making equipment, storing all raw shellfish products in a cooling unit at a temperature of ⩽7°C prior to serving.
This study underscores the need for better protection and monitoring of shellfish-growing areas and vigilance in food-handling practices during harvesting, shipment and food preparation, particularly with foods served raw or undercooked. Based on research demonstrating required temperatures for inactivation of other viruses, including poliovirus, feline calicivirus, murine norovirus and hepatitis A virus (HAV) in bivalve molluscs and other foods, the low temperatures achieved after steaming were probably inadequate to completely inactivate norovirus [Reference Baert18–Reference Kirkland24]. For oysters cooked by frying, baking, stewing, or steaming, poliovirus survival ranged from 7% to 13%, corresponding to only about 90% or 1 log10 reduction [Reference DiGirolamo, Liston and Matches19]. Studies of murine norovirus and HAV viruses in shellfish document little or no inactivation in short cooking times until temperatures are ⩾85 °C [Reference Hewitt and Greening20, Reference Sow21]. In a recent review of processing strategies to inactivate enteric viruses in shellfish Richards et al. [Reference Richards, McLeod and Le Guyader22] note that none of the typical cooking or processing methods can guarantee total virus inactivation without impacting the organoleptic qualities of the shellfish. The UK Ministry of Agriculture, Fisheries and Food recommend that shellfish be heated to at least 90 °C for 90 s to achieve extensive virus inactivation. Previous outbreaks of viral gastroenteritis attributed to cooked oysters provide further evidence that typical cooking procedures are inadequate to completely inactivate the viruses and prevent illness [Reference McDonnell23, Reference Kirkland24]. Therefore, we recommend that minimum temperatures, above the typically recommended temperature of 63°C for 15 s, be required for shellfish products to be sold as steamed in the USA. Additionally, because consumers may assume that any cooked oysters are safe to eat; advisory notices, similar to those posted for eating raw shellfish, should include warnings to patrons, that eating undercooked shellfish may cause illness. This warning is especially relevant for those with underlying health conditions because they are at higher risk for serious illness.
ACKNOWLEDGEMENTS
We thank Ruth Lassiter, Susan Sullivan, Candice A. Yocum, Dianne D. Fore, Jamie N. Tyler and Takashia Penny for assistance with the case-control study, Jennifer Cortes, Doug Esposito, Catherine Yen, and Ben Lopman for help with the household transmission study, and Nicole Gregoricus for laboratory analysis of stools.
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention, North Carolina Division of Public Health, Wake County Human Services, Wake County Department of Environmental Services and the University of North Carolina, Gillings School of Global Public Health.
DECLARATION OF INTEREST
None.





