INTRODUCTION
Non-tuberculous mycobacteria (NTM) are environmental organisms residing in soil and water, with low pathogenicity to humans. Over recent decades, the isolation of NTM, and the disease caused by NTM in both AIDS and non-AIDS populations have been increasingly reported worldwide [Reference Martin-Casabona1–Reference Van Ingen3]. This rise is certainly due to the increase in number and quality of mycobacteriology laboratories, and the improvement of laboratory techniques, as well as to the advent of AIDS in the 1980s. However, there are probably other factors that can justify this trend, such as the larger number of immunocompromised patients surviving today, rising life expectancy and the availability of computed tomography scanning [Reference Polverosi4, Reference Glassroth5].
NTM disease and its pattern of presentation are related to the interactions between species-specific virulence factors and host factors. Although generally NTM virulence is low, it may differ between species and some strains might demonstrate different virulence [Reference Taillard6–Reference Kikuchi8]. Regarding the host, pre-existing structural lung disease [tuberculosis, cystic fibrosis, chronic obstructive pulmonary disease (COPD), silicosis and other pneumoconiosis], alcoholism, smoking, oesophageal reflux and alterations of innate or acquired immunity are acknowledged risk factors for development of NTM pulmonary disease (NTM-PD) [Reference Hoefsloot9, Reference Weiss and Glassroth10].
The NTM infection's clinical presentation is non-specific and in the clinical setting it is not easy to distinguish environmental contamination with transient colonization from pulmonary disease. In order to standardize the definition for NTM-PD and to reach a more accurate diagnosis, in 2007 the American Thoracic Society, in collaboration with the Infectious Disease Society of America (ATS/IDSA), published guidelines for the diagnosis, treatment and prevention of NTM disease. In this guideline, it is specified that the diagnosis of NTM-PD requires the presence of respiratory symptoms, radiological features, microbiological criteria and the exclusion of other diagnoses [Reference Griffith11].
The clinical relevance of NTM species could differ by region or setting and some authors suggest modulation of diagnostic criteria according to clinical relevance of each species [Reference Hoefsloot9, Reference Van12].
Considering the complexity of the NTM-PD diagnosis, due to the difficulty in applying ATS/IDSA criteria in different settings, we collected data on NTM isolates in the mycobacteriology laboratory of our hospital over 2 years, with the aim of contributing to clarifying the application of those criteria in clinical practice and to describing our local epidemiology. We identified patients with positive specimens from the Infectious Disease Unit and the Section of Respiratory Medicine and collected their medical records to classify the patients by the presence of the ATS/IDSA criteria for NTM-PD.
MATERIALS AND METHODS
Data collection
We retrospectively reviewed the database of the mycobacteria isolated in a mycobacteriology laboratory (Tuscany Regional Reference Centre for Mycobacteria, Careggi Hospital, Florence, Italy) for the period from January 2011 to December 2012. We excluded 349 mycobacteria of the Mycobacterium tuberculosis complex and 32 NTM isolated from non-respiratory specimens. Finally, we identified a total of 554 NTM isolated from respiratory samples [sputum, bronchoalveolar lavage (BAL), pleural effusion]. We analysed the epidemiological data of NTM isolates, considering also the demographic characteristic (age, sex, geographical origin) of the patients with positive specimens. In the second part of our study, among the patients with NTM isolates, we selected those admitted to the Infectious Disease Unit and the Section of Respiratory Medicine who had full clinical data available. For each patient we collected clinical and radiological information from clinical records. We define a ‘NTM-PD case’ as a patient fulfilling ATS/IDSA diagnostic criteria (clinical, microbiological and radiological criteria) in the absence of other pulmonary infections. We compared clinical data between patients defined as NTM-PD cases and as NTM-PD colonized, to identify potential risk factors associated with NTM-PD.
Laboratory diagnosis
Specimens were decontaminated using a standard N-acetyl-l-cysteine-NaOH method. A rhodamine-auramine stain method was utilized to analyse specimens for acid-fast bacilli. Cultures were performed on liquid and solid medium at 35–37 °C. As liquid medium, a non-radiometric automated system (BATECT™ MGIT™ 960, Becton Dickinson, USA) was used to detect mycobacterial growth; solid media (Lowenstein–Jensen) were observed weekly for up to 2 months. Positive cultures were microscopically confirmed and identified to differentiate M. tuberculosis complex from NTM using a rapid immunochromatographic test to detect MTbc-specific MPT64 antigen (MGIT TB identification test). A further differentiation up to species level was performed using commercially available molecular genetic methods (line probe assay): Hain GenoType® MTBC for differentiation of the M. tuberculosis complex and Hain GenoType® Mycobacterium CM (Hain Lifescience GmbH, Germany) for non-tuberculous more common mycobacteria. Another Hain kit was used to identify some uncommon species (GenoType® Mycobacterium AS, Hain Lifescience GmbH) [Reference Russo, Tortoli and Menichella13]. In some cases a further sequencing of specific genetic targets was needed in order to differentiate species between a complex (e.g. M. avium complex) or a group (e.g. M. fortuitum group) or to detect some infrequently encountered species [Reference Tortoli14]. The obtained sequences were compared with the GenBank database in order to match the best species identity.
Statistical analysis
Data were analysed using Stata software v. 11.0 (StataCorp, USA). The statistical significance of the differences in quantitative data between the two groups (NTM colonized patients and NTM-PD cases) was determined using the Wilcoxon rank sum test. Pearson's χ 2 test was used for statistical correlations between qualitative variable, applying Fisher's exact test when needed. Differences were considered significant for P values ⩽0·05.
Ethical standards
The ethics committee of Careggi Hospital approved the study (register no. 57/13). The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
RESULTS
Epidemiological data
A total of 554 NTM were isolated from 462 patients, between January 2011 and December 2012. Most (66%) patients were male, with a mean and median age of 64 and 69 years, respectively. The majority of patients came from Europe (413, 89·4%), particularly from Italy (393, 85·1%). The majority of isolates were from sputum specimens (372, 67·1%). Three hundred and ninety-one patients (84·6%) had only one species of NTM isolated from one source, 29 (6·3%) had only one species from two sources and 17 (3·7%) had one species from three different source. Twenty-five patients (5·4%) had two or three different NTM species isolated from ⩾2 sources: 21 (4·6%) had two species from two sources, three (0·6%) had two species from three sources and one (0·2%) had three species from three sources. The most prevalent NTM species were M. xenopi (219, 39·5%), and mycobacteria of the M. avium complex (MAC) (216, 38·9%) followed by the Rapidly Growing Mycobacteria group (39, 7%) (Tables 1 and 2).
Table 1. Microbiological date of the 554 NTM isolated from respiratory sample in the years 2011–2012
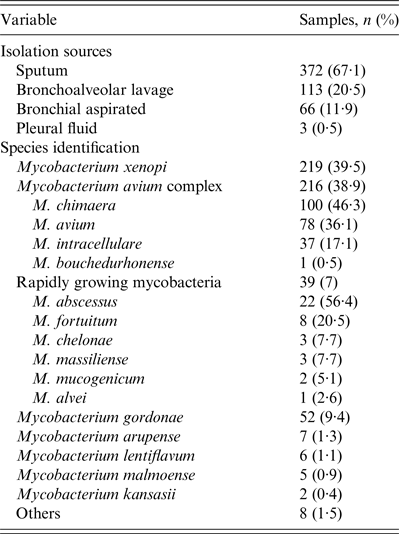
NTM, Non-tuberculous mycobacteria.
Table 2. Epidemiological characteristics of the 462 patients from which the NTM were obtained
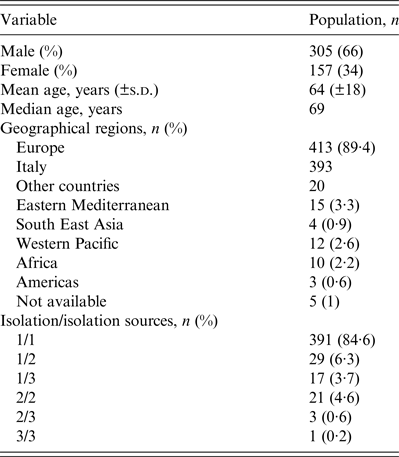
NTM, Non-tuberculous mycobacteria.
Clinical data
A subset of 88 patients, admitted to the Infectious Disease Unit and the Section of Respiratory Medicine, were included for the analysis of clinical data. According to ATS/IDSA criteria, we classified 15 (17%) patients as NTM-PD cases and 73 (83%) as colonized patients. Of these, 10 patients were considered colonized (although they met clinical, microbiological and radiological criteria), because another diagnosis excluded NTM-PD: acute exacerbation of COPD (three patients), tuberculosis (one patient), lung cancer (three patients), pneumonia (one patient), exacerbation of cystic fibrosis (one patient), sarcoidosis (one patient) (Fig. 1). The mean of examined respiratory samples in colonized patients was 3·8.
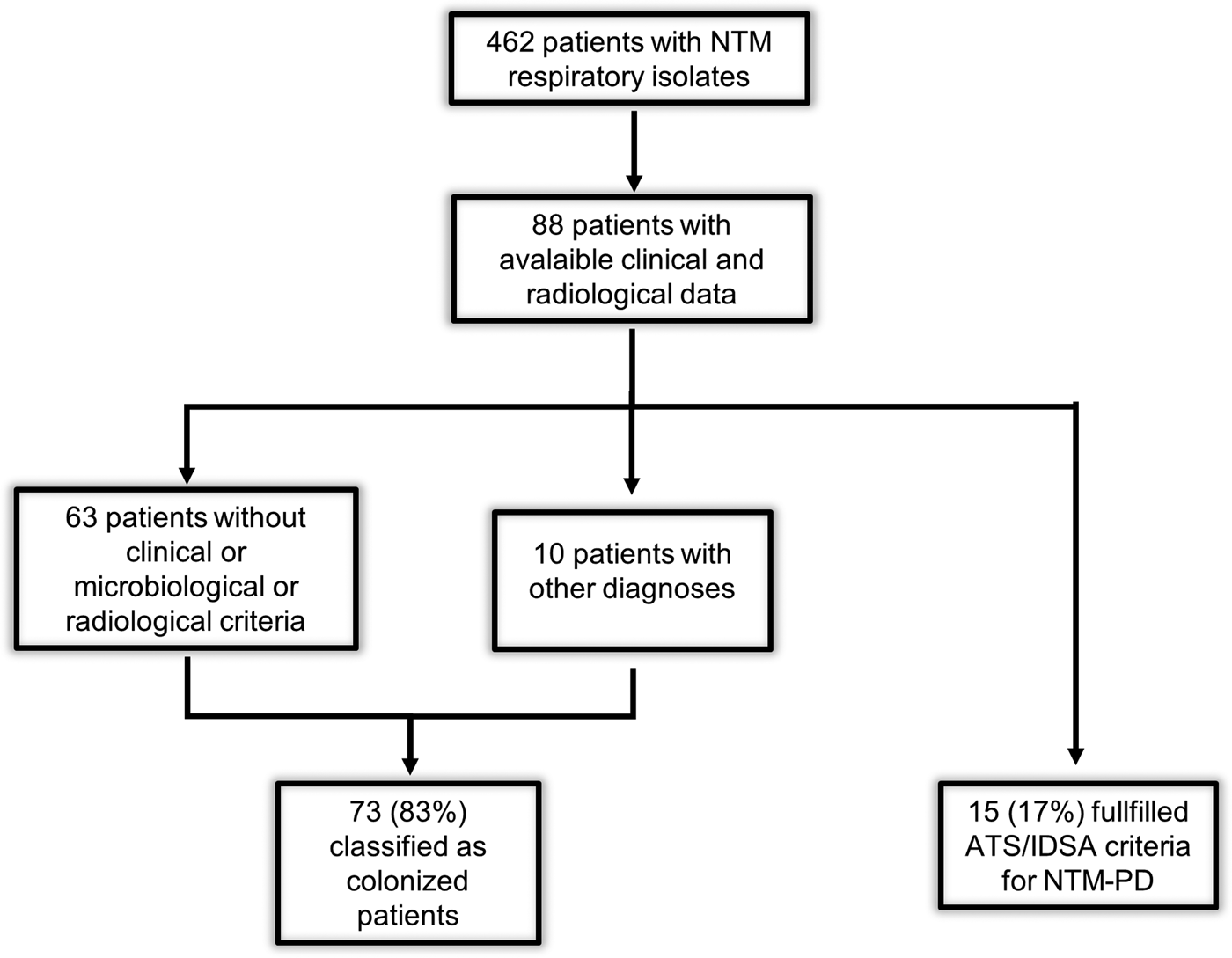
Fig. 1. Flowchart of the study. ATS/IDSA, American Thoracic Society/Infectious Disease Society of America; NTM, non-tuberculous mycobacteria; NTM-PD, non-tuberculous mycobacteria pulmonary disease.
The baseline characteristics of patients are shown in Table 3. Comparing colonized and NTM-PD patients we did not find statistically significant differences of age, gender and comorbidity between the two groups.
Table 3. Baseline characteristics of the 88 NTM-infected patients (colonized or NTM-PD) included in the analysis of the clinical data
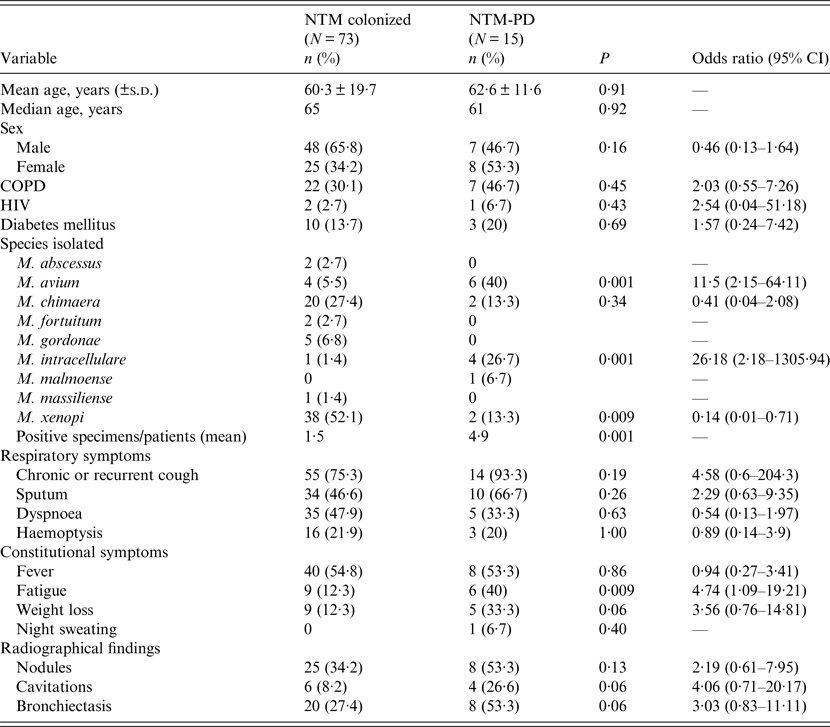
CI, Confidence interval; COPD, Chronic obstructive pulmonary disease; HIV, human immunodeficiency virus; NTM, non-tuberculous mycobacteria; NTM-PD, non-tuberculous mycobacteria pulmonary disease; s.d., standard deviation.
There were more male than female patients colonized by NTM, while NTM-PD appeared to be more prevalent in females.
Comparing the considered symptoms and signs, we found some differences between colonized and NTM-PD patients. However, only fatigue was significantly more prevalent in NTM-PD patients (P = 0·009). Moreover, 14 NTM-PD patients (93·3%) had chronic or recurrent cough, which was a higher percentage than in colonized patients (75·3%). Analysing radiological features, NTM-PD patients presented cavitary lesions at a higher percentage than colonized patients (P = 0·06).
MAC species were prevalent in the 15 NTM-PD patients: M. avium and M. intracellulare were associated with NTM-PD (P = 0·001), whereas M. xenopi was statistically associated with colonization (P = 0·009).
DISCUSSION
Despite the improvements of radiological and microbiological techniques the diagnosis of NTM disease remains a challenging task, due to the difficulty in ascribing clinical significance of NTM isolates from the respiratory tract [Reference Van12].
In 2010 Winthrop stated that it was still necessary to clarify some queries about NTM epidemiology and to determine risk factors for NTM-PD [Reference Winthrop15].
In the past years many laboratories have reported changes in the number and species of NTM isolated from clinical specimens: those isolations changed according to different geographical areas and different species emerged. In European countries increased isolation of M. xenopi has been described [Reference Martin-Casabona1]. We did not study the incidence of species isolated over the years, but we considered the whole number of NTM species isolated between 2011 and 2012 in our laboratory and the most prevalent species was M. xenopi (39·5%), followed by MAC species (38·9%). This finding is in accord with data reported in a recent study by van der Werf et al. [Reference van der Werf16], which collected data from several national tuberculosis reference laboratories of the European Union, although the authors did not specify the source of NTM isolates. In Italy, in the period 2001–2010, these authors reported 2498 NTM isolates obtained from all types of specimens and the prevalent species encountered was M. xenopi (687, 27·5%), followed by M. avium (350, 14%) [Reference van der Werf16].
In our study we applied ATS/IDSA criteria to distinguish NTM-PD patients from colonized patients and we also tried to identify risk factors for NTM-PD. We found only 17% of patients satisfying ATS criteria, a lower percentage than in the study of Del Giudice et al., [Reference Del Giudice17], carried out between December 2006 and September 2009. In this Italian retrospective study, Del Giudice et al. identified 39 patients who fulfilled ATS/IDSA microbiological criteria (two positive cultures from the same mycobacteria species sputum or one positive culture from BAL or transbronchial or lung biopsy). Next, they studied these 39 patients for the presence or not of the other ATS/IDSA criteria (respiratory symptoms, exclusion of other diseases, radiological criteria). Sixteen (41%) patients fulfilled ATS/IDSA criteria [Reference Del Giudice17]. This percentage is higher than those reported in our study and other European studies, probably due to different enrolment criteria. Indeed, our study and those other studies enrolled patients that only had at least one positive sample and did not necessarily satisfy ATS microbiological criteria. A Greek study considered a period from 2007 to 2013 and described 120 patients with at least one positive culture for respiratory NTM isolation. Fifty-six (46%) patients met microbiological criteria, but only 12 (16%) of the patients with adequate clinical data fulfilled the ATS/IDSA criteria [Reference Panagiatou18]. In The Netherlands, van Ingen et al. reviewed retrospectively medical files of patients with positive samples for NTM between January 1999 and January 2005. He found 212 patients with NTM isolates from the respiratory tract of which 53 (25%) met ATS/IDSA criteria [Reference Van Ingen3].
Regarding the symptoms suggestive for NTM-PD, weight loss did not reach the statistically significant association, but the results suggested a trend for a possible association with NTM-PD (P = 0·06); only fatigue was significantly associated with NTM-PD (P = 0·009). Van Ingen et al. reported the same association between fatigue and NTM-PD [Reference Van Ingen3]; however, it is a non-specific symptom burdened by a high risk of recall bias.
High-resolution computed tomography can surely contribute to evaluation of the radiological findings typical for NTM-PD: in our study, we observed that cavitary lesions and bronchiectasis are prevalent in NTM-PD (26·6% and 53·3%, respectively) compared to colonized patients (8·2% and 27·4%, respectively), suggesting a trend of possible association, although the low number of patients did not allow a statistically significant association to be obtained. As is known, two radiological patterns are the most frequent, i.e. thin-walled cavities with surrounding parenchymal opacity in the fibrocavitary lung disease; and multifocal bronchiectasis and small nodules in the mid and lower lung field typical of the non-cavitary pattern. Other radiological features could be ground glass pattern, or single nodule, consolidation. However, in the clinical setting, it is very difficult to relate the radiological findings with NTM-PD. It is important to note that other diseases could show these radiological findings: cavitary lesions can occur in pulmonary malignancy, and in infections due to fungi or Nocardia species. Similarly, the bronchiectasis-nodular type pattern was quite specific for NTM-PD, but other diseases could present with similar lesions (cystic fibrosis, primary ciliary dyskinesia, tuberculosis, nocardiosis, and other diseases that cause bronchiectasis).
In our NTM-PD patients, the most prevalent species were M. avium (six patients, 40%) and M. intracellulare (four patients, 26·7%), and although the number of patients and NTM isolates is low to allow firm conclusions to be made, those two species were significantly associated with NTM-PD (P = 0·001). In the literature, the association between species and NTM-PD is debated: some studies report that MAC is the most common species isolated in patients with definite disease in North America, Asia-Australia and Europe [Reference Koh19–Reference Simons21]. By contrast, some authors observed that no NTM species predicted pulmonary disease [Reference Bodle22]. In our analysis M. xenopi was prevalent in colonized patients with only two cases of NTM-PD, this is in contrast with findings of other studies that report this species as the second cause of disease after MAC [Reference Marras23]. Moreover, a systematic review on M. xenopi pulmonary disease showed 45% of patients with advanced NTM-PD and a high prevalence of males (80%), data that we cannot confirm due to the low number of observed cases [Reference Varadi and Marras24].
With regard to the characteristics of the host, we observed a median age of 65 years in colonized patients and 61 years in NTM-PD patients, without significant difference. Elderly people are more susceptible to NTM-PD and colonization: in other studies the mean age is between 50 and 70 years, without significant difference with colonized patients; as we found [Reference Mirsaeidi25]. A retrospective study carried out in a Canadian population identified risk factors for NTM colonization, comparing NTM-colonized patients with a control group of individuals with culture-negative sputum: by multivariate analysis the predictors of NTM colonization included age ⩾60 years and female sex [Reference Hernandez-Garduno and Elwood26]. Older age was a risk factor for NTM-PD, but it was not able to distinguish disease from colonization.
NTM-PD occurs prevalently in two groups of patients: white middle-aged or elderly men with risk factors (e.g. underlying lung disease including COPD) and elderly non-smoking women without risk factors (e.g. Lady Windermere syndrome) [Reference Reich and Johnson27]. In our study only one woman presented the features of Lady Windermere syndrome and four elderly men had underlying lung disease (COPD), of whom three had exposure to tobacco (data not shown). The other patients could not be categorized into either of those two groups.
In contrast to our data [which report no difference of gender within the NTM-PD group (eight women vs. seven men)], studies in Oregon reported a prevalence of elderly women with NTM-PD [Reference Winthrop2, Reference Cassidy28]. Conversely, European reports show a prevalence of elderly men affected by NTM-PD [Reference Van Ingen3, Reference Del Giudice17]. Our finding could be explained by considering the limited number of patients and the women's median age that was higher than men's median age (67 years vs. 57·6 years, without significant difference). This difference was consistent with the data reported in Oregon.
In the literature, COPD and other pre-existing pulmonary diseases were risk factors for NTM infection [Reference Piersimoni and Scarparo29]. A high percentage of our patients had a diagnosis of COPD, especially in NTM-PD (43·7%). Fowler et al investigated the prevalence of NTM in a cohort of patients with bronchiectasis due to several causes (e.g. Cystic fibrosis transmembrane conductance regulator mutation, idiopathic, immune deficiency) and 10/98 patients presented a positive sputum culture for NTM, not necessarily associated with NTM-PD [Reference Fowler30]. The prevalence and clinical relevance of NTM isolation in COPD was analysed by Hoefsloot et al. in patients presenting with an acute exacerbation of COPD; NTM were isolated from 22% of those patients [Reference Hoefsloot31]. A Danish study reported an association between COPD and NTM-PD, especially when associated with inhaled corticosteroid use [Reference Andrejak32]. Thus the association between COPD and NTM is still not clearly defined.
The main limitations of our study are that it is retrospective and the number of patients with NTM-PD is too low to allow a powerful statistical analysis.
In conclusion, our study confirms the difficulty in discriminating between NTM colonization and NTM-PD using the known risk factors or based on clinical, radiological or microbiological data. Based on the current lack of strong evidence in this field, in clinical practice only the monitoring of colonized patients could allow exclusion of the diagnosis of NTM-PD.
Further prospective studies and new biological markers are needed to identify new tools that can be useful for the diagnosis of NTM-PD, and also to clarify if NTM-colonized patients are at higher risk for the development of pulmonary disease over the course of months or years.
ACKNOWLEDGEMENTS
This study received no financial support.
DECLARATION OF INTEREST
None.







