Infection due to methicillin-resistant Staphylococcus aureus (MRSA) represents a public-health threat in economically developed countries. According to data from the European Antibiotic Resistance Surveillance System, in many European countries there has been a trend of MRSA infection increase in healthcare settings in the years 2000–2005, with frequencies varying from zero in northern Europe to 50% in southern European countries [1].
At one time, MRSA was considered to be primarily a hospital-associated pathogen; however, it has recently emerged as a pathogen in the general community. Several studies have described the rapid spread of a dominant clone of community-acquired MRSA (CA-MRSA) (the so-called USA300 clone) in the USA and Canada in the general population and individual communities [Reference Moran2–Reference Gilbert4]. However, in Europe multiple CA-MRSA clones have been detected in people outside the hospital setting [Reference Tristan5]. CA-MRSA strains often carry the genes for Panton–Valentine leukocidin (PVL), a toxin that causes polymorphonuclear leukocyte lysis and tissue necrosis [Reference Boyle-Vavra and Daum6].
Recently, outbreaks of CA-MRSA skin infections have been reported in men who have sex with men (MSM) [Reference Shastry, Rahimian and Lascher7, Reference Diep8], many of whom were infected with HIV-1 [Reference Lee9]. In particular, CA-MRSA has been shown to cause skin and soft tissue infections in HIV-1-positive MSM who engage in high-risk sexual behaviour and to spread by direct skin-to-skin transmission or by indirect transmission such as a bath, hot tube or sauna [Reference Lee9].
A recent cross-sectional study conducted in San Francisco showed that the prevalence of nasal colonization with CA-MRSA varied from 2·8% in homeless and marginally housed adults to >6% in intravenous drug users and runaway youths [Reference Pan10]. In Europe no study has addressed the prevalence of CA-MRSA colonization in HIV-1-infected MSM. To this end we conducted a case-control study to assess the prevalence of S. aureus and CA-MRSA colonization in outpatient HIV-1-infected MSM and to identify the correlates of colonization.
The study included all HIV-1-infected MSM who consecutively presented for periodical clinical consultation at the HIV-STI Unit of the San Gallicano Institute in Rome, Italy during the period from June 2007 to June 2008. For all participants, a brief standard face-to-face interview was performed to collect information on demographic characteristics, medical history (including infections and hospitalizations), antibiotic use in the previous year, sexual habits and history of sexual/social contacts; virological, immunological and clinical data on HIV-1 infection were also collected. For these variables, individuals who were positive for S. aureus colonization were compared to those who were negative by performing χ2 analysis. Statistical analyses were performed using SPSS software, version 15.0 (SPSS Inc., USA).
To detect signs of skin infection, a total body surface examination was performed and swabs of both anterior nares were taken after having obtained written informed consent from the participant. The swabs were inoculated onto blood agar, salt mannitol agar and ChromID MRSA plates (bioMérieux, France) and incubated at 37°C for 24–48 h. Cultures were examined for growth at 24 h and 48 h and identification of S. aureus was based on conventional tests. Antibiotic susceptibility was determined by the semi-automated system Vitek 2 (bioMérieux). For molecular analysis, genomic DNA was extracted using the Qiamp Mini kit (Qiagen GmbH, Germany) and used as a template in PCR assays. To confirm phenotypic identification, detection of the nuc gene (coding for S. aureus nuclease) and of the mecA gene (coding for methicillin resistance) was performed, as previously described [Reference Monaco11]. The presence of lukS-PV−lukF-PV coding for the two subunits of the PVL toxin was also assayed by PCR [Reference Monaco11].
A total of 104 HIV-1-infected MSM agreed to participate in the study. The median age of participants was 39 years (interquartile range 34–47 years); 16 (15·4%) participants were non-Italians. At enrolment, none of the participants showed any clinical evidence of skin infection. S. aureus colonization was found in 24 participants (23·1%). None of the isolates proved to be MRSA and PVL toxin genes were not detected in any of the isolates.
There was no statistically significant association between S. aureus colonization and demographic, behavioural or other clinical characteristics (Table 1). Nevertheless, colonized patients, compared to non-colonized patients, tended to use more recreational drugs (OR 1·85, 95% CI 0·65–5·63, P=0·23), to have had a sexually transmitted infection (OR 1·94, 95% CI 0·61–7·32, P=0·23) and to report frequent fingernail biting (OR 1·72, 95% CI 0·47–5·70, P=0·33). It is noteworthy that all participants with diabetes were colonized with S. aureus. However, no association was found between colonization and the HIV-1-related clinical, virological, or immunological variables, or with the use of antiretroviral drugs (Table 2). None of the seven participants who had undergone trimethoprim–sulfamethoxazole prophylaxis was colonized with S. aureus.
Table 1. Demographic, behavioural and medical characteristics of 104 HIV-1-infected MSM, according to S. aureus nasal colonization status
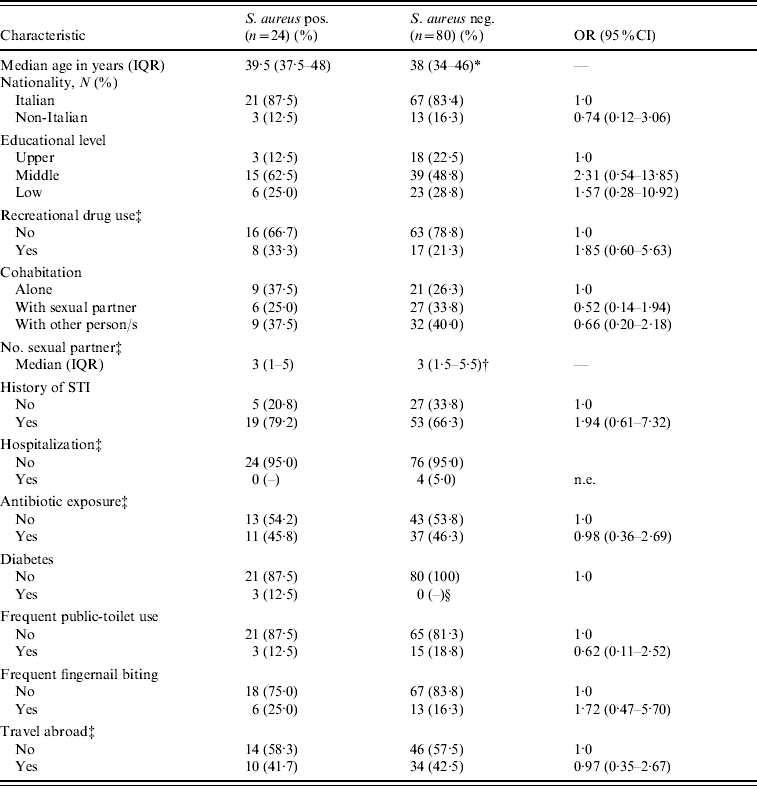
OR, Odds ratio; CI, confidence interval; IQR, interquartile range; n.e., not evaluable; STI, sexually transmitted infection.
* P=0·74.
† P=0·98.
‡ During the previous year.
§ Undefined (P=0·001).
Table 2. HIV-1-related clinical, virological, and immunological characteristics of study participants, according to S. aureus nasal colonization status
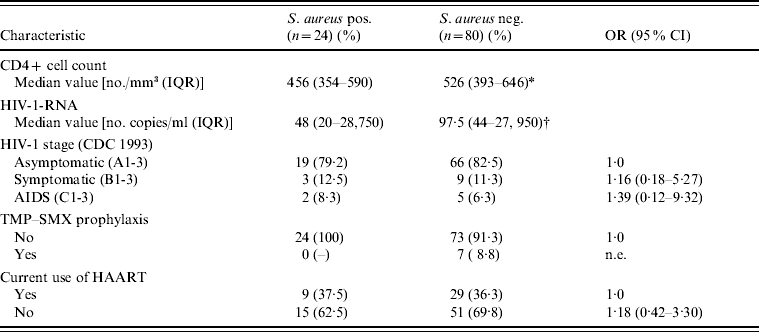
OR, Odds ratio; CI, confidence interval; IQR, interquartile range; TMP–SMX: trimethoprim–sulfamethoxazole; HAART, highly active antiretroviral therapy; n.e., not evaluable.
* P=0·99.
† P=0·75.
Although the study population consisted of individuals who were at high risk for CA-MRSA infection according to the published literature [Reference Shastry, Rahimian and Lascher7–Reference Lee9], neither CA-MRSA skin infection nor colonization were observed. Nevertheless, 23·1% of the study population harboured S. aureus in the nose, a rate corresponding to that observed in healthy individuals [Reference Wertheim12]. Nevertheless, no significant association between S. aureus colonization and severity of immunodeficiency or HIV-1-RNA viral load was found in our population. The lack of MRSA colonization in such a group of subjects thought to be at risk for CA-MRSA infections is unexpected. This finding might be explained by the limited sample size, which reduced the sensitivity of the survey or because sampling was limited to the anterior nares while other common sites of colonization, such as the oropharynx, groin, rectum, and axilla were not investigated. Nevertheless, another study conducted in Italy on 460 patients at hospital admission found only one case (0·2%) of MRSA colonization [Reference Orsi13]. The lack of evidence of CA-MRSA colonization in HIV-1-infected MSM as well as in the general patient population is consistent with the observation that in Italy only a few cases of CA-MRSA infections have been reported to date [Reference Monaco11, Reference Tinelli14].
Different from the situation in Italy, the frequency of isolation of CA-MRSA has increased in groups with a lower than expected risk for colonization in other European countries including southern European countries (Spain, Greece) [Reference Manzur15, Reference Vourli16]. Along with Spain and Greece, Italy shares a high rate of healthcare-associated (HA)-MRSA. The discrepant situation in Italy shows that a high rate of HA-MRSA is not predictive of the frequency of CA-MRSA in the population. This seems plausible since HA-MRSA and CA-MRSA are clonally different, and although HA-MRSA clones have been long established in hospitals and other healthcare facilities [Reference Monaco17], CA-MRSA clones might not yet have emerged in the community. Our study also indicates that there are important epidemiological differences in the incidence of CA-MRSA circulation in the USA and a European country. Thus, data produced in one country cannot be immediately generalized to another and only further locally conducted epidemiological studies can provide an insight into the local circulation of CA-MRSA in both general and selected populations.
ACKNOWLEDGEMENTS
This study was supported in part by grants from the Italian Ministry of University and Research (FIRB 2005 ‘Costruzione di un Laboratorio Nazionale per lo Studio delle Resistenze Batteriche agli Antibiotici’), and from the Ministry of Labor, Health and Social Services, CCM project ‘Laboratory-based surveillance of infections due to antibiotic-resistant bacteria’. The authors thank Mr Mark Kanieff for his assistance in revising the manuscript.
DECLARATION OF INTEREST
None.




