INTRODUCTION
Bone marrow (BM) examination is a useful method for establishing a diagnosis in patients with or without human immunodeficiency virus (HIV) infection who present with fever of unknown origin (FUO) and cytopenia [Reference Hot1, Reference Benito2]. The diagnostic yield of BM examination varies from 18% to 47%, and the most common diagnoses made by BM examination are haematological malignancies and infections, especially those caused by mycobacteria [Reference Hot1–Reference Riley6]. Mycobacterial BM infection is a disseminated infection associated with a high mortality rate and prompt anti-mycobacterial treatment is crucial to achieve a good outcome [Reference Wang7, Reference Chou8]. However, timely identification of mycobacterial species is not simple because mycobacterial culture methods are time consuming and a substantial proportion of mycobacterial infections are culture-negative [9–Reference Griffith11]. Therefore, empirical therapy for tuberculosis (TB) is often instituted pending bacteriological confirmation of mycobacterial species [Reference Blumberg10, Reference Griffith11].
In countries with a high burden of TB, mycobacterial BM infections in HIV-positive patients are almost always caused by Mycobacterium tuberculosis (MTB) [Reference van Schalkwyk, Opie and Novitzky3, Reference Khandekar12]. Therefore, the empirical use of anti-TB treatment is a rational decision [Reference Blumberg10]. However, in countries with a low prevalence of TB, the case numbers of BM infections due to M. avium complex (MAC) and those due to MTB in HIV-positive patients are similar [Reference Benito2, Reference Pacios13]. Furthermore, in the USA, a country with a very low prevalence of TB, the majority of mycobacterial BM infections in HIV-infected patients are caused by MAC [Reference Luther5, Reference Riley6, Reference Northfelt14].
In Taiwan, where TB is endemic, the prevalence of TB decreased during 2000–2009, while the prevalence of non-tuberculous mycobacterial (NTM) infections increased [Reference Lo15, Reference Lai16]; in addition, the prevalence of HIV infection is very low in Taiwan [Reference Yang17]. There is a lack of studies regarding mycobacterial BM infections in Taiwan. Moreover, previous studies have shown limited knowledge about mycobacterial BM infections and discrimination between MTB and NTM BM infections is difficult. Since a better understanding of mycobacterial BM infections could be vital to ensuring that these patients receive timely and accurate anti-mycobacterial treatment, we performed a retrospective cross-sectional study to investigate the profile of HIV-infected and non-HIV-infected patients with mycobacterial BM infections in which histopathological and microbiological BM studies were performed at a medical centre in northern Taiwan, and to compare the clinical characteristics between patients with MTB and NTM BM infections.
PATIENTS AND METHODS
Patient population and definitions
This study was performed at a 2500-bed, university-affiliated tertiary hospital in northern Taiwan. From 1 January 2001 to 31 December 2009, data of BM specimens sent for mycobacterial culture and histological examinations in the microbiology and pathology laboratories were retrieved from the computerized databases. During the 9-year study period, the mean annual incidences of TB, NTM disease and HIV infection in the study hospital were 391, 193, 106 cases per year, respectively. All patients with BM aspirates with a positive mycobacterial culture or histopathological specimens showing acid-fast bacilli (AFB) and/or granulomatous inflammation were analysed. BM is regarded as one of the involved sites in disseminated mycobacterial infections, and the gold standard for diagnosing mycobacterial BM infection is a positive BM mycobacterial culture [Reference Wang7, Reference Lai18]. However, a substantial proportion of mycobacterial infections are culture-negative; therefore, a clinical diagnosis can be established based on histopathological features that are characteristic of mycobacterial infection combined with characteristic clinical presentations, such as miliary pattern, fibrocavitary, nodular or bronchiectatic lesions on the chest radiographs and presence of lymphocytosis in pleural fluid or cerebrospinal fluid (CSF) [9, Reference Griffith11].
In this study, both microbiological and histopathological criteria were adopted. Mycobacterial BM infection was diagnosed if the patient fulfilled any of the following criteria: (1) isolation of mycobacteria from BM culture; (2) histopathological demonstration of granulomatous changes and AFB from BM with negative stains for fungi; and (3) histopathological demonstration of granulomatous changes or AFB from BM plus a culture-proven diagnosis or a clinical diagnosis of mycobacterial infection in body sites other than BM and without concomitant fungal infections. Clinical diagnoses of MTB and NTM infections in other body sites were made as described above.
Mycobacteriology studies
All specimens sent for mycobacterial culture in the study hospital were processed according to previous description and guidelines [Reference Lai18–Reference Tan20]. NTM isolates were identified to the species level using conventional biochemical methods. Drug susceptibility testing to first-line anti-TB drugs including isoniazid, rifampicin and ethambutol was performed using the modified proportional disk elution method.
Histopathological examination of BM biopsy specimens
BM samples were obtained by posterior iliac crest trephine biopsy. Histopathological changes were assessed by pathologists and the routine stains for microorganisms included Gomori methenamine-silver stain, periodic acid-Schiff stain, and Kinyoun acid-fast stain [Reference Ker21].
Clinical characteristics and outcome
Information on age, sex, underlying immunocompromised conditions, haematological tests, extent of mycobacterial infection, types of specimens positive for mycobacteria, histopathological findings from BM, and in-hospital mortality were collected by chart review and further analysed. In addition to a positive mycobacterial culture, mycobacterial infection in other body sites was also recorded based on the presence of relevant laboratory, histopathological or radiological findings. Underlying immunocompromised conditions, including diabetes mellitus, HIV infection, solid-organ cancer, haematological disorders, liver cirrhosis, autoimmune disease, and end-stage renal disease (ESRD) requiring dialysis were recorded. Patients without HIV serological testing were presumed to be HIV negative if they had no past history to suggest HIV infection. Leukopenia was defined as leukocyte count of <4000 cells/mm3, thrombocytopenia was defined as platelet count of <150000 cells/mm3, and anaemia was defined as haemoglobin level of <13 g/dl for men or <12 g/dl for women. Pancytopenia was defined as the presence of leukopenia, thrombocytopenia, and anaemia. The 90-day survival rate was evaluated and that of patients discharged from the hospital within 90 days was investigated using medical records of subsequent outpatient department follow-up visits.
Statistical analysis
Continuous variables are expressed as mean±standard deviation (s.d.) when there was a parametric distribution. Otherwise, data are expressed as median [interquartile range (IQR)]. Student's t test or Mann–Whitney U test were used to compare continuous variables. We used Fisher's exact test or χ 2 test to compare proportions; besides odds ratios (ORs) and their 95% confidence intervals (CIs) were calculated. 90-day survival curves of MTB, and NTM BM infections were compared using the Kaplan–Meier method and the log-rank test. P values <0·05 were considered to be statistically significant. All analyses were performed with SPSS, version 11.0 (SPSS Inc., USA).
RESULTS
Clinical characteristics
From January 2001 to December 2009, pathological examinations were performed in 1191 BM biopsy samples and a total of 693 BM specimens were sent for mycobacterial culture. Mycobacterial BM infection was diagnosed in 24 patients (Fig. 1), which was equivalent to 4·0 cases/100 000 inpatients and their characteristics are given in Table 1. Of these, 15 (five TB, 10 NTM), two (one TB), and seven (five TB) were diagnosed as mycobacterial BM infections based on the first, second and third criteria mentioned above, respectively. Fever (83%) was the most common reason of BM examination, followed by cytopenia (38%). Sixteen (67%) patients were men and the mean age of all patients was 53·7 ± 16·9 years. BM was the sole infection site in four (17%) patients and the most frequent concomitant infection foci were lung (n = 16, 67%), bloodstream (n = 9, 38%), abdomen (n = 9, 38%), and central nervous system (CNS) (n = 4, 17%). Nine (38%) of the patients were HIV positive. Of the remaining 15 patients, 12 (80%) had a negative serological test for HIV infection and the remaining three (20%) had no past history to suggest HIV infection. Other underlying immunocompromised conditions included diabetes mellitus (n = 4, 17%), liver cirrhosis (n = 3, 13%), malignancy (n = 3, 13%), ESRD (n = 1, 4%), systemic lupus erythematosus (n = 1, 4%) and myelodysplastic syndrome (n = 1, 4%); six (25%) patients had no immunosuppressed condition.
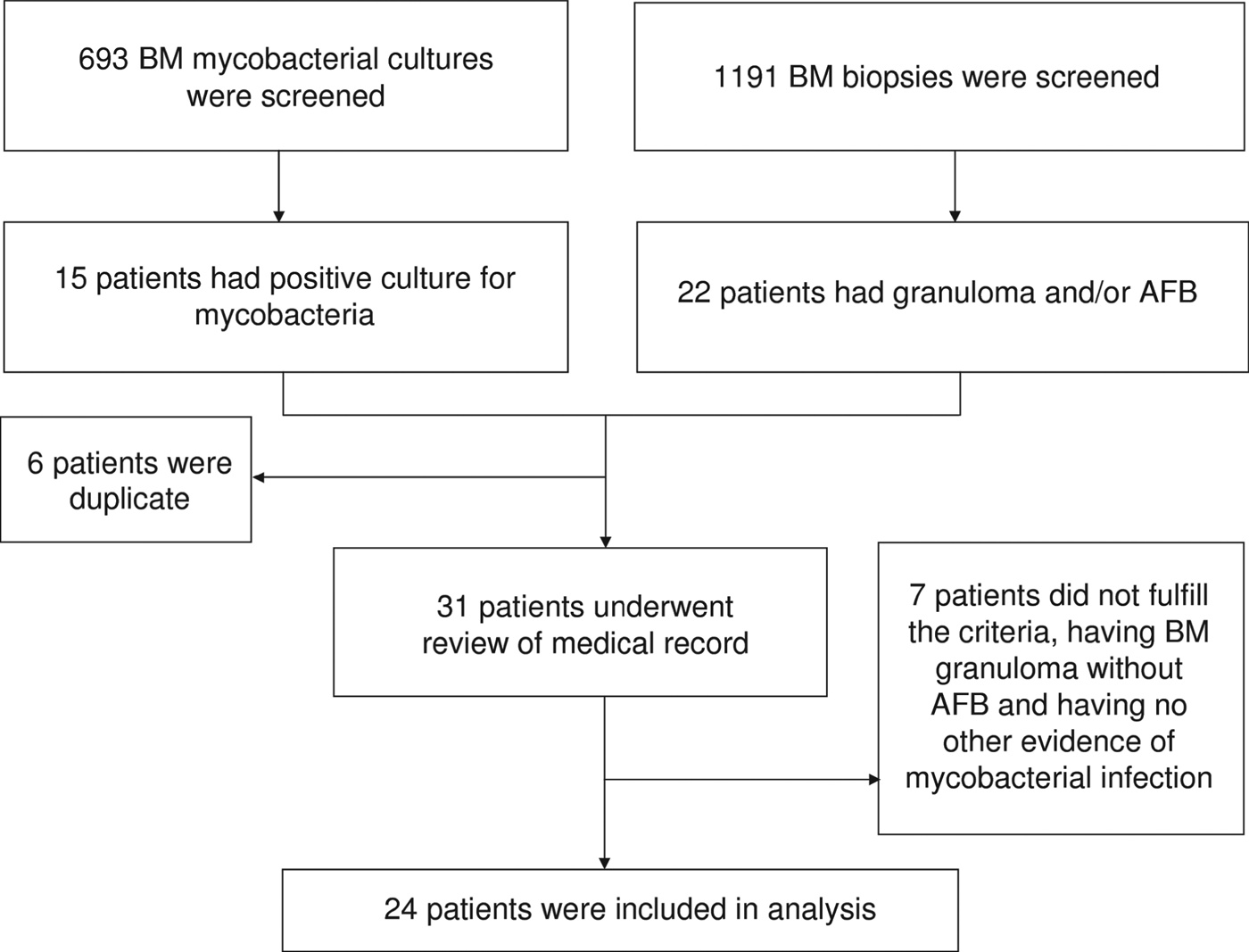
Fig. 1. Fluxogram of identification of patients. BM, bone marrow; AFB, acid-fast bacilli.
Table 1. Characteristics of 24 patients with mycobacterial BM infection, 2001−2009
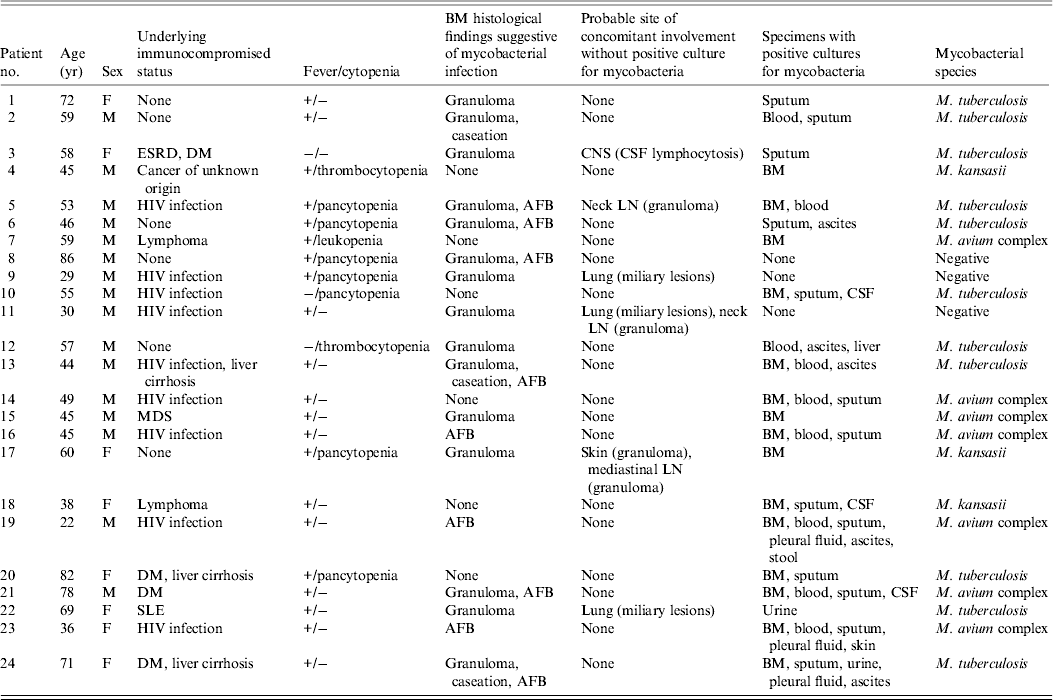
BM, Bone marrow; ESRD, end-stage renal disease; DM, diabetes mellitus; CNS, central nervous system; CSF, cerebrospinal fluid; HIV, human immunodeficiency virus; AFB, acid-fast bacilli; LN, lymph node; MDS, myelodysplastic syndrome; SLE, systemic lupus erythematosus.
MTB isolates were obtained from 11 (46%) patients and NTM species were isolated from 10 (42%) patients, including MAC in seven and M. kansasii in three patients. Three (27%) patients with MTB infection and four (40%) with NTM infection were HIV positive (OR 0·6, 95% CI 0·09–3·5, P = 0·66). None of the three patients with infection due to M. kansasii were HIV positive. At least one set of mycobacterial blood culture was performed for all patients. Bloodstream infection was found in four (37%) patients with MTB infection and in five (50%) with NTM infection (OR 0·6, 95% CI 0·1–3·3, P = 0·67); lung involvement was found in eight (73%) patients with MTB infection and in six (60%) with NTM infection (OR 1·8, 95% CI 0·3–11·1, P = 0·66); abdomen involvement was found in six (55%) patients with MTB infection and in three (30%) with NTM infection (OR 2·8, 95% CI 0·5–16·9, P = 0·39); and CNS involvement was found in two (18%) patients with MTB infection and in two (20%) with NTM infection (OR 0·9, 95% CI 0·1–7·9, P = 0·65). Patients with MTB BM infection were significantly older than those with NTM infection (60·5 ± 11·7 vs. 47·7 ± 15·3 years, P = 0·043).
Outcome
All MTB isolates were susceptible to rifampicin and isoniazid. All patients with MTB or unspecified mycobacterial infection were initially treated with a four-drug combination anti-TB treatment (isoniazid, rifampicin, pyrazinamide, ethambutol), and patients with NTM infection received standard antibiotic treatment [Reference Griffith11]. MAC infections were treated with a macrolide (clarithromycin or azithromycin) in combination with ethambutol, or rifampicin, or rifabutin. M. kansasii infection was treated with an ethambutol-based combination regimen. The all-cause in-hospital mortality rate was 33%. No significant difference in clinical characteristics was noted between patients who died and those who survived (Table 2). The Kaplan–Meier survival curves for patients with MTB and NTM BM infections are shown in Figure 2, and the 90-day survival rates were 68% and 60%, respectively (log-rank P = 0·61).

Fig. 2. The 90-day Kaplan–Meier survival curves of bone marrow infections due to non-tuberculous mycobacteria (NTM) and M. tuberculosis (MTB) during 2001–2009 (hazard ratio 0·68, 95% confidence interval 0·15–3·0, P = 0·61, log-rank test).
Table 2. Comparison of characteristics between survivors and non-survivors
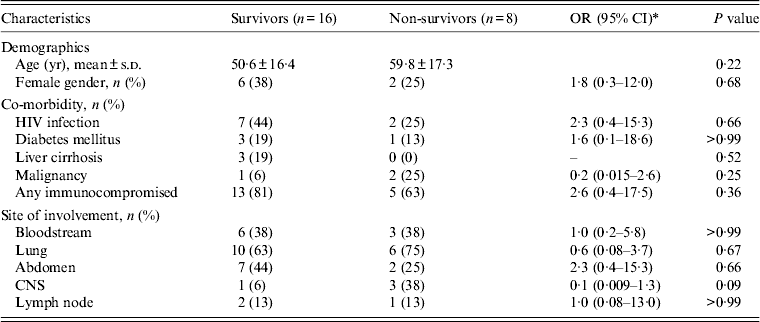
OR, Odds ratio; CI, confidence interval; s.d., standard deviation; HIV, human immunodeficiency virus; CNS, central nervous system.
* Odds ratio is not calculable due to zero frequencies in some cells.
BM examinations
Granulomas were seen in 15 (63%) patients, and only three (13%) had caseation. BM mycobacterial culture was positive in 15 (63%) patients and AFB was seen in nine (38%). Of the nine patients with a negative BM mycobacterial culture, all had granulomas while AFB was seen in only two (22%) patients. Of the 15 patients with a negative blood culture for mycobacteria, eight (53%) had a positive BM culture for mycobacteria. Of the seven patients with negative BM and blood mycobacterial cultures, three had positive mycobacterial cultures from other body sites, two had a clinical diagnosis of miliary TB, and one had BM granuloma plus AFB. Of the nine patients with a positive blood culture for mycobacteria, seven (78%) had a positive BM culture for mycobacteria. A positive blood mycobacterial culture was not associated with a significantly higher positive rate of BM mycobacterial culture (OR 3·1, 95% CI 0·5–19·9, P = 0·39). BM was the only body site in which mycobacteria were isolated in four (17%) patients (MAC, n = 2; M. kansasii, n = 2).
A comparison of haemograms and BM examinations between patients with MTB and those with NTM BM infections is shown in Table 3. There were no significant differences in haemograms between the two groups of patients. However, the proportion of patients with granulomas was higher in the group of patients with MTB infections than in patients with NTM infections (82% vs. 30%, P = 0·030). Furthermore, caseation was observed exclusively in patients with MTB infections. All patients with NTM infections had BM cultures positive for mycobacteria and 46% of patients with MTB infections had BM cultures positive for mycobacteria (P = 0·012).
Table 3. Comparison of patients with BM infections due to MTB and those due to NTM by haemogram and BM findings
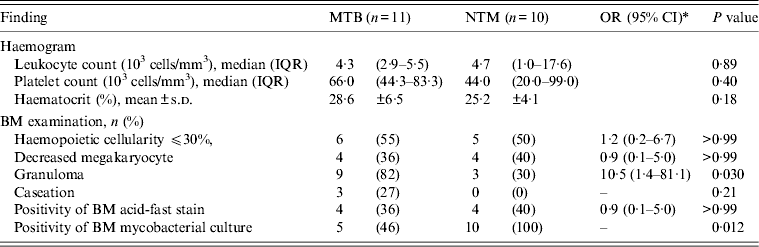
BM, Bone marrow; MTB, M. tuberculosis; NTM, non-tuberculous mycobacteria; OR, odds ratio; CI, confidence interval; IQR, interquartile range; s.d., standard deviation.
* Odds ratio is not calculable due to zero frequencies in some cells.
DISCUSSION
In this hospital-based study in Taiwan we found that a substantial proportion of patients with mycobacterial BM infections were HIV negative or without any pre-existing immunocompromised condition. We also found that the number of NTM BM infections was similar to that of MTB infections and that in addition to MAC, M. kansasii was an important pathogen in non-HIV-infected patients. NTM and MTB BM infections were associated with similar outcomes and most deaths occurred within 30 days after diagnosis. Further, patient's age and histological features of BM biopsy specimens, such as granulomas and caseation, might be helpful in distinguishing between NTM and MTB BM infections before final identification of mycobacterial species.
Hafner et al. showed that in patients with disseminated MAC infection, BM bacterial load was 1000-fold higher than bacterial load in blood and was correlated with poor outcome [Reference Hafner22]. In our study, culture-positive rates of BM and blood were 63% and 38%, respectively; the combination of both had a positive rate of 71%. Since the combination of BM and blood cultures provide the maximal diagnostic yield for disseminated mycobacterial infections, the superiority of BM culture to blood culture was not always found in previous studies [Reference Pacios13, Reference Ker21, Reference Kilby23]. In the present study, mycobacterial species were isolated from BM in 8/15 patients with negative blood cultures. The additional diagnostic value of BM culture might be partially explained by the lack of association between BM and blood mycobacterial load [Reference Hafner22]. Therefore, the importance of BM cultures should not be overlooked in patients with suspected disseminated mycobacterial infections.
Although previous studies have shown that the diagnostic value of BM culture is superior to that of BM histopathology for the detection of mycobacterial BM infections, BM histopathology nonetheless remains a valuable diagnostic tool [Reference Benito2, Reference Luther5, Reference Northfelt14, Reference Nichols24, Reference Akpek25]. In patients with disseminated mycobacterial infection, early treatment has been shown to be associated with better survival [Reference Wang7, Reference Chou8]; however, it is difficult to distinguish between NTM and MTB BM infections before culture results are available. Nichols et al. found that caseous necrotic granulomas were observed exclusively in patients with MTB BM infections and that BM granulomas in patients with BM infections due to MTB tended to be larger and more tightly cohesive than those in patients with BM infections caused by MAC [Reference Nichols24]. Benito et al. also showed that caseous necrosis was observed only in BM infections due to MTB and that BM granulomas were observed in 83·3% of patients with BM infections caused by MTB and in 64·3% of patients with BM infections due to MAC [Reference Benito2]. In the present study, caseation was noted in 3/11 patients with MTB BM infections and was not observed in any of the patients with BM infections due to NTM. In addition, the prevalence of BM granulomas was significantly higher in patients with MTB BM infections than in patients with BM infections caused by NTM. Further investigations utilizing polymerase chain reaction or interferon-γ assays to aid in the early differentiation of MTB from NTM BM infections are warranted [Reference Escobedo-Jaimes26, Reference Lai27].
Studies investigating the utility of BM examinations in HIV-positive patients have demonstrated a positive relationship between the proportion of MTB in mycobacterial BM infections and the regional TB incidence density [Reference Benito2, Reference van Schalkwyk, Opie and Novitzky3, Reference Luther5, Reference Riley6, Reference Khandekar12–Reference Northfelt14]. Unlike NTM infections, a clinical diagnosis of TB is generally accepted if there is good radiological or histopathological evidence of TB in patients who respond well to treatment [Reference Blumberg10, Reference Griffith11]. In countries with a high prevalence of TB, patients with suspected disseminated mycobacterial disease are usually assumed to have TB and receive early anti-TB treatment [Reference Rose28]. Although TB incidence has declined in Taiwan, the rate of NTM infections has increased [Reference Lo15, Reference Lai16]. We found that in HIV-positive patients, the potential for BM infections caused by MAC might be similar to that caused by MTB. Therefore, caution is warranted when initiating empirical anti-TB therapy based on results of BM histopathological examinations before a culture-proven diagnosis is established.
Mycobacterial BM infection is relatively uncommon in HIV-negative patients. A study in the USA found that mycobacterial species were isolated from only 2% of BM specimens from HIV-negative patients with FUO compared to 20% from HIV-positive patients [Reference Riley6]. Similarly, a study in France found that only 1·5% of HIV-negative patients with FUO had BM infections caused by MTB [Reference Hot1]. Furthermore, studies have shown that mycobacterial BM infections in HIV-negative patients are almost always caused by MTB [Reference Hot1, Reference Riley6, Reference Pacios13, Reference Rose28]. In our study, the numbers of mycobacterial BM infections in non-HIV-infected and HIV-infected patients were 15 and nine, respectively. Moreover, 40% of mycobacterial BM infections in non-HIV-infected patients were caused by NTM. These findings may most likely be attributed to the relatively low HIV prevalence in Taiwan [Reference Yang17]. Additionally, our findings were in agreement with the increasing secular trend of NTM infections and the emergence of disseminated NTM disease in Taiwan [Reference Chou8, Reference Lai16]. In our study, six patients were considered to be immunocompetent and in addition to MAC, M. kansasii was another important pathogen of BM infections in HIV-negative patients. These findings imply that mycobacterial BM infections should be considered in HIV-negative patients as well as in immunocompetent hosts.
Our study has several limitations. First, the retrospective nature of the study might underestimate the incidence of mycobacterial BM infections because the decision to perform BM examinations was at the physicians' discretion. Second, in Taiwan a consent form must be obtained before HIV testing and in our study three patients with a low index of suspicion did not receive HIV tests. Third, this study was conducted in a tertiary referral hospital in northern Taiwan. Therefore, the data are not fully representative of the general population. Forth, the relatively small number of patients in this study limited the statistical power. Fifth, there are miscellaneous causes of BM granuloma, such as infections, sarcoidosis, malignancies, and drugs [Reference Brackers de Hugo29]. Therefore, a diagnosis of mycobacterial BM infection without a positive mycobacterial BM culture result would be erroneous. Given the probability of culture-negative mycobacterial infections [9–Reference Griffith11], this bias would be minimized by the coexistence of BM AFB or mycobacterial infections at other body sites. Finally, a clinical diagnosis of TB, rather than NTM infection, would be made without positive cultures and the comparison between MTB and NTM BM infections might be further biased by the conception that more MTB BM infections were diagnosed according to the second and third criteria.
In summary, MTB and NTM BM infections had similar frequencies in patients hospitalized in the tertiary-care hospital in Taiwan. In addition to MAC, M. kansasii was another important agent of NTM in HIV-negative patients. A comprehensive evaluation including regional TB epidemiology, patients' characteristics and immune status, and BM histopathological findings are imperative in the management of mycobacterial BM infections.
DECLARATION OF INTEREST
None.







