INTRODUCTION
Cryptosporidiosis is a gastrointestinal illness characterized by watery diarrhoea, which may be accompanied by abdominal cramps, loss of appetite, low-grade fever, nausea, vomiting, and weight loss [Reference Chen1, Reference Nichols, Fayer and Xiao2]. The causal agents, species of the genus Cryptosporidium, are chlorine-resistant, protozoan parasites that are transmitted by the faecal–oral route when Cryptosporidium oocysts are ingested through the consumption of contaminated food or water or through person-to-person or animal-to-person contact [Reference Chen1–Reference Yoder and Beach4]. There are multiple Cryptosporidium species; Cryptosporidium hominis is a species that is predominantly transmitted in humans [Reference Xiao, Ryan, Fayer and Xiao5]. Cryptosporidium species have emerged as a major cause of outbreaks of diarrhoeal illness because of their environmental hardiness [Reference King and Monis6] and chlorine resistance [Reference Korich7]. As a result, the parasite has been implicated in outbreaks associated with drinking water [Reference Yoder8], recreational water [Reference Yoder9, Reference Beach, Fayer and Xiao10], food [Reference Beach, Fayer and Xiao10–15], and child-care programmes [Reference Turabelidze16–Reference Tangermann19].
In August 2007, the Colorado Department of Public Health and Environment (CDPHE) detected a marked increase in the number of reported cases of cryptosporidiosis without an identified common exposure. By late August 2007, CDPHE had received 56 reports of cryptosporidiosis for the month when the expected number of reported cases in Colorado for August was 12. CDPHE, local public health agencies, and CDC conducted an investigation with the objective of identifying risk factors for sporadic transmission that were associated with this large statewide increase in the incidence of cryptosporidiosis.
METHODS
Case finding
For this statewide investigation, a confirmed case was defined as a Colorado resident who had a positive Cryptosporidium laboratory stool test in June–October 2007. Reports of confirmed cases of cryptosporidiosis were entered by laboratories, hospitals, and local public health agencies into the Colorado Electronic Disease Reporting System (CEDRS).
Case-control study
For the case-control study, we defined a confirmed case of cryptosporidiosis as gastrointestinal disease in a Colorado resident with a positive Cryptosporidium laboratory stool test and onset of gastrointestinal symptoms from 1 August 2007 to 17 September 2007. Gastrointestinal symptoms, defined as diarrhoea (⩾3 loose or watery stools in 24 h), vomiting, or other symptoms such as abdominal (non-menstrual) cramping, belching, bloating, or gas production. Since cases of cryptosporidiosis tend to be clustered within households and because the case-control study was aimed at identifying risk factors for illness in the community, the laboratory-confirmed case was required to have had the earliest onset date in the household. A control was defined as a Colorado resident who had experienced no gastrointestinal symptoms from 1 August 2007 to time of interview (15 September–3 November 2007). Two controls were matched by age range (0–<5 years, 5–<12 years, 12–<18 years, and ⩾18 years) and geographic area (based on case-patient's telephone number) for each case-patient.
Controls were identified by sequential digit dialling using the phone number of the case-patient as the starting number. The number ‘1’ was sequentially added to the starting phone number until the caller identified and interviewed the first control, and then the number ‘1’ was sequentially subtracted from the starting phone number until the caller identified and interviewed the second control. In addition, phone numbers collected during the 2007 FoodNet Population Survey, a previous randomized, population-based phone survey, were used to facilitate the identification of controls in the Denver metropolitan area (Adams, Arapahoe, Boulder, Broomfield, Denver, Douglas, and Jefferson counties). Some participants of the FoodNet Population Survey had previously agreed to participate in future studies to help public health officials prevent and control transmission of infectious agents. As with the case-patients, only one person per household was interviewed as a control.
In the study, a standardized questionnaire was developed and administered by telephone. Case-patients and corresponding matched controls were asked about possible exposures during the 2 weeks prior to the case-patient's onset of symptoms. These risk exposures included food and water consumption, recreational water exposure, child care and household exposures, farm and animal contact, person-to-person contact, and travel history during the exposure period as well as basic demographics.
Data were entered into an electronic database using Access 2003 (Microsoft Corporation, USA). All analyses of investigation data were performed using SAS, version 9.1 (SAS Institute Inc., USA). Odds ratios (OR) were calculated using conditional logistic regression to account for matching. Variables with a univariate OR P value <0·20 were included in a larger multivariable model and assessed using stepwise regression analysis. χ2 test or Fisher's exact test was used to compare demographic characteristics between cases and controls. Two-sample t tests were used to examine differences between subgroups of case-patients. Two-sided P values <0·05 were considered statistically significant.
Laboratory investigation
Testing for Cryptosporidium was performed at the CDPHE state laboratory using direct immunofluorescent assay (DFA) (MeriFluor® Cryptosporidium/Giardia, Meridian Bioscience Inc., USA). Positive specimens were confirmed by CDC using the formalin-ethyl acetate concentration technique and the Merifluor® Cryptosporidium/Giardia DFA. Fresh bulk stool samples from confirmed case-patients were sent to CDC for speciation/genotyping and subtyping by PCR and DNA sequencing. DNA was extracted from washed stool specimens using the FastDNA Spin Kit for Soil (Qbiogene Inc., USA) and Cryptosporidium species/genotypes were identified based on PCR–RFLP analysis of the small subunit (SSU) rRNA gene as described previously [Reference Xiao20]. Cryptosporidium-positive specimens were subtyped by DNA sequencing of the 60-kDa glycoprotein gene (GP60) [Reference Sulaiman21].
RESULTS
Case finding
Through surveillance, we identified 160 Colorado residents who had a positive Cryptosporidium laboratory stool test from June 2007 to October 2007. Figure 1 depicts these laboratory-confirmed cases by date of symptom onset as well as the average annual cryptosporidiosis case counts reported by CDPHE for the same time period in 2004–2006. A peak in 2007 cases occurred during mid- to late-August and late September, which exceeded baseline levels of case reporting by CDPHE from 2004 to 2006.
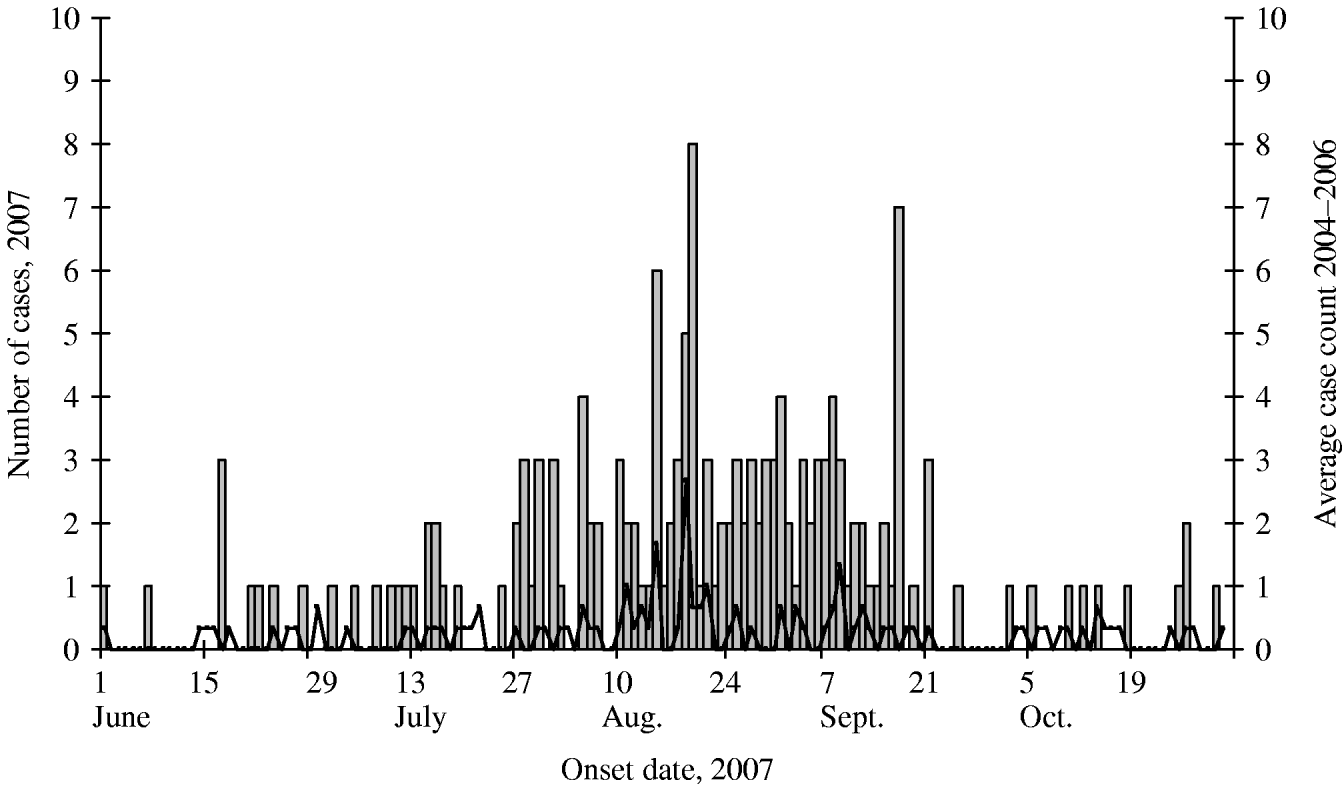
Fig. 1. Laboratory-confirmed cases by date of symptom onset during the statewide increase (n=160) and average case counts for 2004–2006 (–––).
Case-control study
Ninety persons met the case-control study inclusion criteria (earliest household onset of gastrointestinal illness from 1 August 2007 to 17 September 2007). Controls were not contacted for eight case-patients and 25 case-patients were not enrolled due to unsuccessful calling attempts. Seven cases had an onset of illness during this time period but the exact onset date was unknown and three case-patients refused to participate. As a result, we enrolled 47 (52·2%) case-patients, of whom 24 (51·1%) were adults (median 19·0 years, range 10 months–68 years) and 23 (48·9%) were female; the majority of the case-patients were white (n=42, 89·4%). Eight of the case-patients were Hispanic (17·4%). The 47 case-patients resided in 15 counties throughout Colorado. Ninety-two matched controls were interviewed for the case-control study. Controls differed significantly from enrolled case-patients only in ethnicity [eight (18·7%) of 46 case-patients vs. four (4·3%) controls were Hispanic, P=0·02].
Of the 47 enrolled case-patients, the most commonly reported symptoms were diarrhoea, tiredness or fatigue, and abdominal cramps (Table 1). The median length of time that case-patients were ill before seeking health care was 4·0 days (range 1·0–27·0 days). Case-patients had a median of 10·0 watery stools in a 24-h period (range 2·0–40·0 stools) with diarrhoea lasting a median of 14·0 days (range 3·0–41·0 days).
Table 1. Clinical symptoms reported by enrolled case-patients
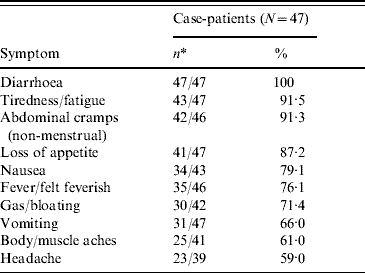
* Denominator varies due to missing or unknown responses.
All of the case-patients consulted their healthcare provider about their illness. The majority of case-patients visited their healthcare provider's office for treatment (n=36, 76·6%) and five (10·9%) case-patients were hospitalized, all but one of whom were adults. Those hospitalized had a median length of hospitalization of 4·0 days (range 2·0–9·0 days). Over half of case-patients used over-the-counter anti-diarrhoeal medications (n=24, 55·8%) and 62·2% (n=28) were prescribed antibiotics or anti-parasitic drugs. About one-third were prescribed nitazoxanide (n=14, 29·8%). No significant difference was noted in the duration of diarrhoea of those prescribed nitazoxanide (n=11; median 17·0, range 6·0–41·0 days) compared with those prescribed other antibiotic or anti-parasitic medications (n=7; median 12·0, range 8·0–16·0 days) (P=0·05); however, case-patients prescribed nitazoxanide had a significantly longer duration of diarrhoea than case-patients who were not prescribed any medications for cryptosporidiosis (n=10; median 11·5, range 4·0–18·0 days) (P=0·02). In addition, no significant differences were noted in age or gender between those prescribed nitazoxanide and those prescribed any other prescription medications or no medications for cryptosporidiosis.
The univariate analyses of individual exposure variables are summarized in Table 2. Cryptosporidium infection was significantly associated with attending child care outside the home or having children in child care outside the home during the 2 weeks before illness [matched odds ratio (mOR) 2·5, 95% confidence interval (CI) 1·1–5·6]. Contact with a child in child care or a child in diapers was also a significant risk factor for infection (mOR 2·5, 95% CI 1·2–4·9). Consumption of produce obtained from a farm or farm stand had a protective effect (mOR 0·3, 95% CI 0·1–0·8). Exposure to untreated drinking water from a lake, river, or stream (mOR 3·3), any recreational water exposure (mOR 1·8), and attendance at a social event (mOR 0·5) approached significance at P<0·15. The results of the final multivariable model are summarized in Table 3.
Table 2. Univariate conditional analysis of exposures in case-patients and controls
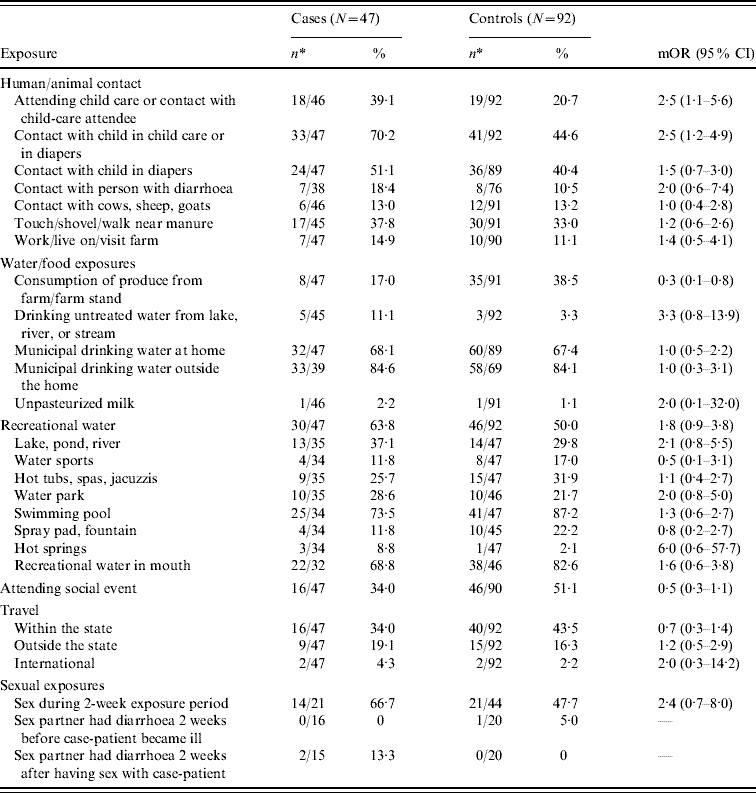
mOR, Matched odds ratio; CI, confidence interval.
* Denominator may vary due to missing or unknown responses.
Table 3. Multivariable conditional analysis of exposures in case-patients and controls
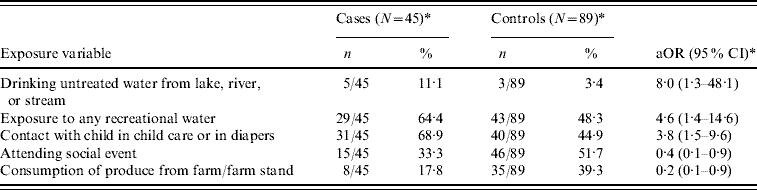
aOR, Adjusted matched odds ratio; CI, confidence interval.
* Two cases and three controls not included due to missing responses.
Laboratory investigation
Seventeen stool specimens from case-patients identified during case finding were analysed. Thirteen (76·5%) of these specimens were positive for Cryptosporidium by DFA. Genotyping identified C. hominis in 11 (100%) of the PCR-positive individuals. Two specimens were not genotyped due to low numbers of Cryptosporidium oocysts. Subtyping analysis identified three distinct subtypes: IaA28R4 (n=8), IbA10G2 (n=2), and IaA12R4 (n=1). There was no geographic clustering by subtype.
DISCUSSION
CDPHE documented an increase in reporting of cryptosporidiosis cases in 2007 compared to baseline reporting from 2004 to 2006. Risk factors for sporadic transmission associated with the statewide increase in cryptosporidiosis reporting in Colorado in 2007 included drinking untreated water from a lake, river, or stream; exposure to recreational water; and contact with children in child care or diapers. The finding of multiple risk factors, the lack of epidemiological links in cases, the geographic dispersion of case-patients, as well as the identification of three different parasite subtypes, suggests at least three separate chains of transmission for Cryptosporidium in Colorado rather than a single source of infection. Our understanding of cryptosporidiosis in the USA, like other gastrointestinal diseases, is based on outbreaks [Reference Jones22], many of which are associated with swimming pool use. However, this study underscores that sporadic transmission involves multiple risk factors, in addition to recreational water use, and complements knowledge derived from previous outbreak investigations. These findings emphasize the need for public health officials to continue broad education of the public about cryptosporidiosis so that they work to reduce Cryptosporidium transmission through exposures not as commonly implicated during investigations of cryptosporidiosis outbreaks, such as drinking untreated water and child-care programme use.
Although recreational water appears to be the major transmission route for Cryptosporidium, based on outbreak reports, cryptosporidiosis outbreaks occurring in child-care programmes have been well documented [Reference Turabelidze16–Reference Tangermann19]. Once Cryptosporidium is introduced into the child-care programme, it can be transmitted easily to other children and staff, and infectious oocysts can easily contaminate surfaces or be transmitted during water-related activities involving inflatable pools, water tables, etc. [Reference Cordell and Addiss23]. The risk of infection in child-care workers has been shown to be greatest in those caring for children in diapers [Reference Tangermann19, Reference Cordell and Addiss23]. Child-care staff and parents should be educated regarding the symptoms of cryptosporidiosis, how Cryptosporidium is transmitted and how to prevent transmission, outbreak control policies, requirements for exclusion, and needed improvements in hygiene [Reference Cordell and Addiss23, 24]. In several instances during this investigation, public health agencies learned of children who had continued to attend child care while ill with diarrhoea, which reinforces the need for enforcing diarrhoea-exclusion policies for child-care attendees. Child-care workers ill with cryptosporidiosis should be reassigned to duties that minimize opportunities to transmit Cryptosporidium (e.g. non-food preparation activities such as administrative duties) [25].
The multivariable analysis found that drinking untreated water from a lake, river, or stream was also a risk factor for infection. The public needs to be educated about the health risks of drinking untreated water from lakes, rivers, or streams and safe and effective treatment methods. Health education materials, camping and recreation advice, and water filtration products should include information advising that to remove Cryptosporidium oocysts, water filters with a pore size of ⩽1 μm should be used. Wording such as ‘absolute 1 μm filter’ should be used and looked for by consumers vs. ‘nominal 1 μm’ filters since filters that have ‘absolute 1 μm filters’ will more consistently remove Cryptosporidium [26].
This study also found that exposure to recreational water venues was a risk factor for infection. This has been observed in many cryptosporidiosis outbreaks, particularly in the summertime, so that Cryptosporidium is now the leading cause of recreational water-associated outbreaks of gastrointestinal disease [Reference Yoder9, Reference Yoder27]. This finding reinforces the need for public health and the aquatics sector to collaborate in educating the public about the risks of cryptosporidiosis and partnering with the public to keep this chlorine-resistant pathogen out of the water.
Eating produce from a farm or farm stand was a protective factor, which supports findings observed in other case-control studies [Reference Hunter28–Reference Roy30]. One explanation for this protective effect may be that continued consumption of contaminated produce may provide protection against overt cryptosporidiosis by keeping antibody levels raised since antibody to the parasite appears to protect from subsequent illness rather than re-infection [Reference Casemore31]. In addition, this protective effect may be explained by an increased mucous layer resulting from increased dietary fibre that decreases infectivity by causing physiological changes that reduce the parasite's ability to attach to the intestinal mucosa [Reference Leitch32]. Attendance at social events, such as picnics, county fairs, parties, or other social gatherings, was also a protective factor against infection. The reasons behind this protective effect are unclear. The limited data in this study showed the same or longer duration of diarrhoea in patients treated with nitazoxanide [Reference Fox and Saravolatz33, Reference Anderson and Curran34] compared to those who received other antimicrobial treatment or were untreated. Although worth further investigation, the small sample size and incomplete knowledge of patient clinical presentation (e.g. were those treated with nitazoxanide more severely ill?) makes it difficult to fully evaluate the drug's efficacy.
The results of molecular testing of Cryptosporidium isolated from stool samples demonstrated that C. hominis was the species infecting case-patients (indicative of human-to-human transmission) and subtype IaA28R4 was the most frequently isolated subtype (found in 72·7% of specimens analysed). However, it must be remembered that only a small percentage of case-patients submitted specimens to public health officials for molecular typing analysis so these results must be interpreted with caution. Prior to 2007, subtype IaA28R4 was an uncommonly identified C. hominis subtype and was implicated as the cause of only two swimming pool-associated outbreaks, one in Ohio (2005) and the other in South Carolina (2006) [Reference Xiao, Ryan, Fayer and Xiao5]. In late summer and autumn of 2007; however, it was implicated as the cause of two swimming pool-associated outbreaks, one in Pennsylvania and one in Idaho, and was widely identified in stool specimens from patients with apparently sporadic cases in Idaho, Iowa, and New Mexico collected during a period of heightened cryptosporidiosis surveillance.
This investigation had a number of limitations. First, enrolment only included 47 case-patients from around the state. Although there was sufficient statistical power to ascertain the risk factors mentioned above, other less common risk factors may not have been detected due to insufficient statistical power. Second, questionnaires were administered to some case-patients several weeks after illness and to some controls several weeks after the exposure period of interest, which may have resulted in recall bias. Although case-patients and controls were asked to recall similar time periods in the past, differential bias could have occurred because case-patients may have reflected on that period of time more carefully because of their illness. Third, only a small number of stool samples were received which limited the ability to perform molecular speciation/genotyping and subtyping to further understand sporadic transmission.
The findings of this statewide case-control study suggest that the multiple risk factors identified for sporadic Cryptosporidium transmission in Colorado in 2007 are similar to those implicated in investigations of outbreaks. This serves as a reminder that while recreational water is a major risk factor for summertime infection, investigations of increased reporting of cases with summertime onset dates should also examine other potential risk factors. This study underscores the need for public health education of healthcare providers, the public, campers and hikers, aquatics staff, and child-care operators regarding prevention and control of cryptosporidiosis when drinking untreated water, when using recreational water venues, or in child-care settings.
ACKNOWLEDGEMENTS
We gratefully acknowledge all of the staff at CDPHE and the Colorado local public health agencies who helped with the administration of our questionnaires and collection of stool specimens. Technical assistance was provided by John Williamson, Sc.D., and Theresa Dearen, B.S., Division of Parasitic Diseases, National Center for Zoonotic, Vector-Borne, and Enteric Diseases, CDC. Phone call assistance was provided by CDC staff, Office of Workforce and Career Development. All funding for this evaluation was provided by the Centers for Disease Control and Prevention.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
DECLARATION OF INTEREST
None.






