INTRODUCTION
Bloodstream infection (BSI) caused by Pseudomonas aeruginosa is a serious and life-threatening condition, especially in immunocompromised hosts and populations with predisposing conditions [Reference Kang1–Reference Wisplinghoff4]. P. aeruginosa is also one of the main organisms responsible for drug-resistant nosocomial infections, being the third most frequently isolated pathogen from blood culture among Gram-negative rods and the seventh among all pathogens [Reference Wisplinghoff4, Reference Giske5]. These infections occur more frequently in patients with severe immunodeficiency, older age, previous antimicrobial therapy, and presence of central venous catheter (CVC) or urinary device [Reference Falagas and Kopterides6, Reference Schechner7]. In addition to being intrinsically resistant to several antimicrobial agents, P. aeruginosa can acquire resistance to multiple (or even all) antibiotics, and multidrug-resistant (MDR) strains, which were first reported in patients with cystic fibrosis, are increasing in frequency [Reference Giske5, Reference Falagas and Kopterides6, Reference Aloush8, Reference Tam9]. The incidence of MDR P. aeruginosa infections is associated with increased morbidity, mortality, and cost [Reference Giske5]. Several studies have attempted to identify risk factors associated with infections caused by antimicrobial-resistant P. aeruginosa strains [Reference Falagas and Kopterides6, Reference Aloush8, Reference Defez10]. However, to our knowledge, few studies have focused on risk factors for BSI caused by MDR P. aeruginosa [Reference Falagas11].
We aimed to identify the factors associated with the isolation of MDR P. aeruginosa strains and evaluate the clinical treatment response and factors associated with mortality in patients with P. aeruginosa BSI.
METHODS
Setting and study design
A retrospective study was conducted in two Italian university hospitals offering a full range of clinical services and admitting about 55 000 patients per year: Catholic University Hospital, a 1600-bed hospital located in Rome, and San Martino University Hospital, a 1500-bed hospital located in Genoa.
The microbiology laboratory database was used to identify adult patients hospitalized from 1 January 2006 to 31 December 2007 with P. aeruginosa BSI, which was defined as the presence of at least one positive blood culture and clinical features compatible with systemic inflammatory response syndrome [Reference Russell12]. Each patient was included in the study only once, at the time of the initial positive blood culture.
Data were extracted from patients' medical records and computerized hospital databases according to a pre-defined questionnaire. Patients were compared regarding demographics, medical history, clinical and laboratory findings, treatment, and outcome of infection. The impact of comorbidities was determined by the Charlson Comorbidity Index [Reference Charlson13], and the overall severity of the patient's illness was rated by the Acute Physiology and Chronic Health Evaluation (APACHE) III score [Reference Knaus14] calculated on the basis of available clinical data for the first 24 h following BSI onset. A case-case-control study design was used. The first case group was composed of patients from whom MDR P. aeruginosa strains were isolated (the MDR P. aeruginosa group); the second case group contained patients from whom non-MDR P. aeruginosa were isolated (the non-MDR P. aeruginosa group). The same control group was used for both case groups. The control group consisted of patients (total of cases:controls=1:2) with no evidence of BSI or positive clinical cultures for P. aeruginosa during their hospitalization and chosen at random from lists of patients admitted to the same ward during the same time period.
Patients were included only if a complete data series could be obtained from their medical records. The distributions of the case and control admissions throughout the study period were similar.
We conducted a two-part analysis. First, a case-case-control study [Reference Harris15, Reference Tumbarello16] in which groups 1 and 2 were compared to controls to determine factors associated with the isolation of MDR and non-MDR strains of P. aeruginosa, respectively. Second, a cohort study including all patients with P. aeruginosa BSI (group 1 plus group 2) was performed to identify factors associated with in-hospital mortality associated with P. aeruginosa BSI using death within 21 days of the first positive blood culture as the outcome and comparing survivors and non-survivors.
Definitions
Prior to data analysis, MDR P. aeruginosa BSI was defined by blood-culture positivity (at least one specimen) for a strain of P. aeruginosa resistant to at least one drug of ⩾3 of the following anti-pseudomonal agents: β-lactams/inhibitors (piperacillin-tazobactam), cephalosporins (cefepime, ceftazidime), carbapenems (imipenem, meropenem), quinolones (ciprofloxacin, levofloxacin), and aminoglycosides (amikacin, gentamicin). Polymyxins-only-susceptible (POS) P. aeruginosa BSI was defined as being caused by a P. aeruginosa strain resistant to all commercially available drugs except colistin.
BSI onset was the date of collection of the first blood culture yielding the study isolate (index culture). BSI source was an infection at a distant site caused by a microbial strain identical to the bloodstream isolate documented by microbiological and physician findings. Infections were defined as nosocomial if the index blood culture had been drawn more than 48 h after admission [Reference Garner17]. Early-onset infections (i.e. cultures drawn within the first 48 h of hospitalization) were classified as healthcare-associated or community-acquired in accordance with the definitions of Friedman et al. [Reference Friedman18].
Septic shock was defined as sepsis associated with organ dysfunction, accompanied by persistent hypotension following volume replacement [Reference Russell12]. An absolute neutrophil count (ANC) <500 neutrophils/μl at the onset of infection was defined as neutropenia.
An in-patient stay of ⩾2 days during the 12 months preceding the index hospitalization was considered prior hospitalization. The use of any antimicrobial for more than 48 h during the 3 months preceding the index admission was considered prior antimicrobial therapy. Antibiotic use was examined, both in terms of whether any antibiotics were used and by aggregate classes (fluoroquinolones, oxyimino-cephalosporins, aminoglycosides, carbapenems, β-lactam-β-lactamase inhibitors). Antibiotic treatment empirically prescribed before in vitro susceptibility test results were available was defined as initial antibiotic treatment and considered ‘inadequate’ [inadequate initial antibiotic treatment (IIAT)] when treatment with an antibiotic possessing in vitro activity against the isolated pathogen was absent.
Microbiology analysis
Bloodstream isolates were identified at the species level using the VITEK 2 (bioMérieux Inc., USA) and/or Phoenix (Becton Dickinson Microbiology Systems, USA) systems. Minimum inhibitory concentrations (MICs) of amikacin, cefepime, ceftazidime, ciprofloxacin, colistin, gentamicin, imipenem, levofloxacin, meropenem, and piperacillin-tazobactam were determined by E-test (AB Biodisc, Sweden). P. aeruginosa (ATCC 27853) and Escherichia coli (ATCC 25922 and 35218) were included as quality-control strains in all sessions. The MICs were classified according to the Clinical Laboratory Standards Institute (CLSI) guidelines [19].
Statistical analysis
Continuous variables were compared by Student's t test if normally distributed and the Mann–Whitney U test if non-normally distributed. Categorical variables were evaluated using χ2 or the two-tailed Fisher's exact test. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated to evaluate the strength of any association. Values for continuous and categorical variables are expressed as mean±standard deviation (s.d.) or percentage of the group from which it is derived, respectively.
Logistic regression was used to identify independent risk factors. Variables associated with BSI caused by MDR P. aeruginosa in the univariate analysis (P⩽0·1) were included at model entry, and a backward stepwise approach was used to identify independent risk factors. Variables were retained in the final model if the P value was ⩽0·05. With the same methodology a second multivariate model was performed for BSI caused by non-MDR P. aeruginosa. The Kaplan–Meier method was used for the survival analysis. Two-tailed tests were used to determine significance; P<0·05 was considered significant. All statistical analyses were performed using the Intercooled Stata program, version 8 for Windows (Stata Corporation, USA).
RESULTS
Incidence and population characteristics
P. aeruginosa BSI was diagnosed in 109 of the 212 658 patients hospitalized in our centres (111 907 in Rome, 100 751 in Genoa) over the 2 years of the study (overall incidence 0·51/1000 admissions). Three patients were not included in the analysis because of a lack of sufficient data, resulting in a final number of 106 cases. There were 212 patients included as controls.
Antimicrobial susceptibility
Of the 106 strains of P. aeruginosa evaluated, 50 (47·2%) displayed resistance to ceftazidime, 48 (45·3%) to cefepime, 47 (44·3%) to levofloxacin, 46 (43·4%) to ciprofloxacin, 44 (41·5%) to imipenem, 42 (37·7%) to meropenem, 38 (35·8%) to gentamicin, 20 (18·8%) to piperacillin-tazobactam, eight (7·5%) to amikacin, and none to colistin. Forty-six strains (12·5%) were classified as MDR P. aeruginosa. Eight isolates (7·5%) were POS.
Risk factors for MDR and non-MDR P. aeruginosa BSIs
Descriptive characteristics of patients with MDR and non-MDR P. aeruginosa BSI are shown in Table 1. The results of a comparison of the case groups with the controls by univariate analysis are shown in Table 2. MDR and non-MDR P. aeruginosa cases had longer hospital stays and previous BSI; previous surgery was more common in these groups. Compared to controls, patients were more likely to have undergone invasive procedures: mechanical ventilation, urinary catheterization, CVC, nasogastric tube, surgical drainage tube, total parenteral nutrition, and have a neutrophil count <500/mm3; previous treatment with corticosteroid and antibiotic therapy with β-lactam/β-lactamase inhibitors, oxyimino-cephalosporins, and carbapenems was more frequent in the patient group.
Table 1. Clinical characteristics of patients
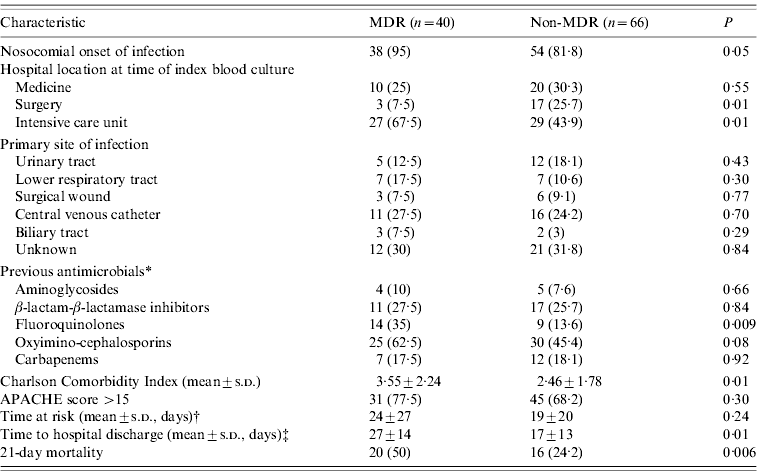
MDR, Multidrug resistant.
Values are n (%) unless otherwise noted.
* Within the 30 days preceding infection onset.
† Duration of hospitalization prior to the date of collection of the first blood culture yielding the study isolate (index culture), calculated only for patients with nosocomial infections.
‡ For patients alive at day 21 (n=70) and calculated from the date of collection of the first blood culture yielding the study isolate (index culture).
Table 2. Univariate analysis of risk factors for P. aeruginosa infection
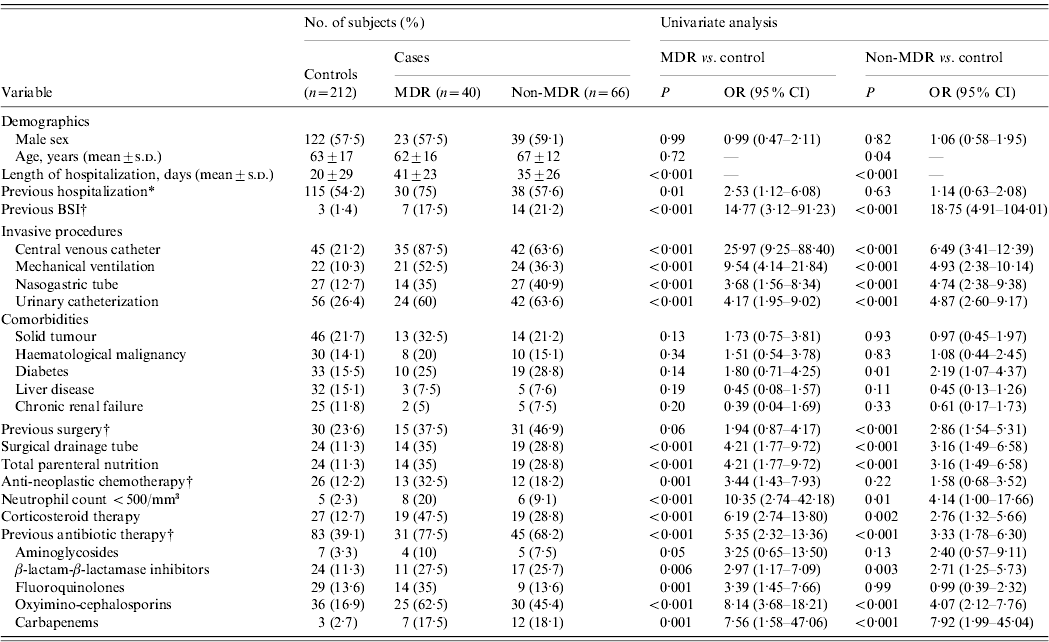
BSI, Bloodstream infection; MDR, multidrug resistant; OR, odds ratio; CI, confidence interval.
Values are n (%) unless otherwise noted.
* Within the 12 months preceding infection onset.
† Within the 30 days preceding infection onset.
Isolation of MDR P. aeruginosa was also associated with previous use of fluoroquinolones or aminoglycosides, previous hospitalization and prior use of anti-neoplastic chemotherapy. The non-MDR P. aeruginosa cases were also significantly older than the controls and were more likely to have diabetes.
In logistic regression analysis, the only three variables independently associated with the isolation of MDR P. aeruginosa were: presence of CVC, previous antibiotic therapy, and corticosteroid use. Independent significant risk factors for the isolation of non-MDR P. aeruginosa were previous BSI, neutrophil count <500/mm3, urinary catheterization, and the presence of CVC (Table 3).
Table 3. Logistic regression analysis of risk factors for P. aeruginosa infection
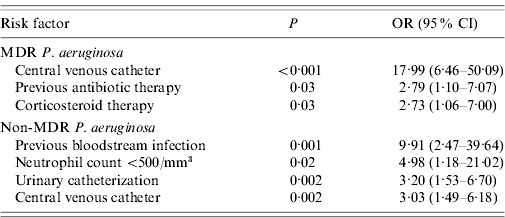
MDR, Multidrug resistant; OR, odds ratio; CI, confidence interval.
Treatment and outcome
Forty patients with MDR P. aeruginosa BSI were empirically treated with the following antimicrobials: oxyimino-cephalosporins (ceftazidime or cefepime; n=7, 17·5%), β-lactam/β-lactamase inhibitor combination (amoxicillin-clavulanic acid or piperacillin-tazobactam; n=15, 37·5%), carbapenems (imipenem or meropenem; n=11, 27·5%), aminoglycosides (amikacin or gentamicin; n=5, 12·5%), fluoroquinolones (ciprofloxacin or levofloxacin; n=7, 17·5%), or others (n=1, 2·5%). Patients infected with non-MDR P. aeruginosa were empirically treated with oxyimino-cephalosporins (n=17, 25·7%), β-lactam/β-lactamase inhibitor combination (n=16, 24·2%), carbapenems (n=15, 22·7%), aminoglycosides (n=8, 12·1%), fluoroquinolones (n=16, 24·2%), or others (n=2, 3·1%). In some cases, the antimicrobials were used in combination therapy. In most cases, the choice in empirical regimen was made by the physician in charge of the patient, and protocols were not used.
In vitro susceptibility testing revealed that the initial therapy was inadequate in 33·9% of the cases (36/106), 12 of which received oxyimino-cephalosporins (33·3%), six fluoroquinolones (16·6%), eight carbapenems (22·2%), nine β-lactam/β-lactamase inhibitor combination (25%), and two aminoglycosides (5·5%). Three patients (8·3%) did not receive antibiotic therapy within 48 h of the blood culture draw. The percentages of patients receiving IIAT were 52·5% (21/40) and 24·2% (16/66) in those with MDR and non-MDR P. aeruginosa BSIs, respectively (P=0·003).
The overall 21-day mortality rate of all patients was 33·9% (36/106). The mortality rate was significantly higher in the MDR P. aeruginosa BSI group than the non-MDR group (55·5% vs. 28·5%, P=0·006). The survival curve also shows that the MDR P. aeruginosa BSI group had a lower probability of survival than the non-MDR group (Fig. 1).
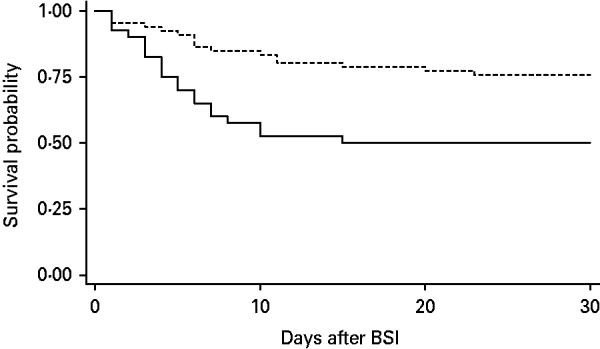
Fig. 1. Survival curve using the Kaplan–Meier method for patients with bloodstream infections (BSI) caused by multidrug-resistant (MDR) P. aeruginosa compared to those with non-MDR P. aeruginosa. The MDR group (——) has lower probability of survival than the non-MDR group (- - -) (P=0·003).
Risk factors for mortality
The results of the univariate and multivariate analyses of risk factors for mortality are shown in Table 4. Univariate analysis revealed significant differences between the survivor and non-survivor subgroups. A significantly higher percentage of non-survivors were older, had a higher mean Charlson Comorbidity Index score, received prior antibiotic and corticosteroid therapy, and had nosocomial infections. Non-survivors were also more frequently in the intensive care unit (ICU), had BSI presentation that included septic shock, and their infections were more frequently caused by MDR P. aeruginosa. A higher percentage of non-survivors received IIAT.
Table 4. Risk factors associated with 21-day mortality
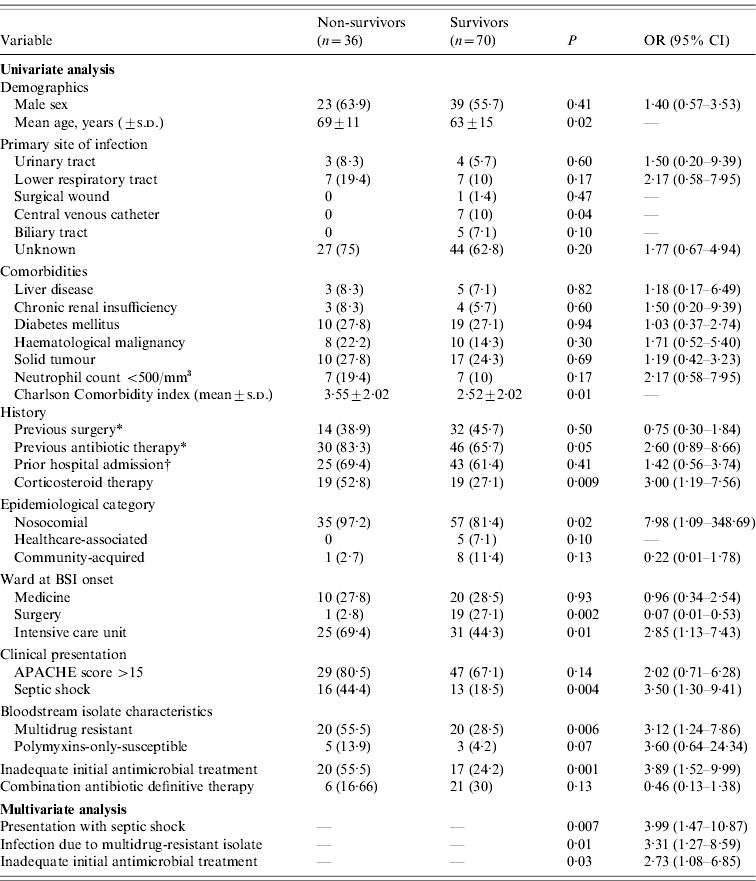
OR, Odds ratio; CI, confidence interval.
Values are n (%) unless otherwise noted.
n=106 patients with P. aeruginosa bloodstream infection (BSI).
* During the 30 days preceding the index blood culture.
† During the 12 months preceding the index hospitalization.
In logistic regression analysis, the variables independently associated with 21-day mortality were presentation with septic shock, infection due to MDR P. aeruginosa, and IIAT.
DISCUSSION
In the present study, we found P. aeruginosa BSI to be a relatively rare condition with an overall incidence of 0·51/1000 admissions, which is similar to that recently reported by Marchaim et al. [Reference Marchaim20].
The prevalence of antimicrobial-resistant P. aeruginosa is increasing in ICU patients [Reference Falagas and Kopterides6]. A large proportion (67·5%) of our patients with MDR P. aeruginosa BSIs were in the ICU at the time of BSI onset. In general, one out of every four cases included in our study was hospitalized in the ICU. This fact may have contributed to the high rate of MDR P. aeruginosa observed in our patients.
Schechner et al. recently demonstrated that independent predictors of P. aeruginosa bacteraemia were severe immunodeficiency, age >90 years, antimicrobial therapy within the previous 30 days, and presence of a CVC or urinary device [Reference Schechner7], but the risk factors for MDR P. aeruginosa were not separately reported. In our study, a case-case-control study design was used, which involves two parallel case-control studies with a common control group, providing a more precise estimate of the risk of a particular P. aeruginosa isolate being MDR. This information is useful as a guide for selecting empirical antibiotic treatments and early institution of contact precautions. By logistic regression, we found that MDR P. aeruginosa BSIs are associated with previous antibiotic therapy, corticosteroid therapy, and CVC.
Exposure to antibiotics predisposes patients to colonization with P. aeruginosa strains that are intrinsically resistant to these agents. P. aeruginosa also has the capacity to rapidly become resistant during the course of anti-pseudomonal drug treatment [Reference Carmeli21]. Therefore, previous therapies increase the risk of infection with selected resistant strains [Reference Falagas and Kopterides6, Reference Gasink22, Reference Van Delden23]. In our experience, MDR strains are isolated more frequently from patients previously treated with different classes of antibiotics: cephalosporins, carbapenems, fluoroquinolones, and β-lactam/β-lactamase inhibitor combinations. In contrast, carbapenems and fluoroquinolones were the antibiotics most frequently related to MDR resistance in previous studies [Reference Falagas and Kopterides6]. Previous exposure to a particular antibiotic is not only associated with the acquisition of resistance to that antibiotic [Reference El Amari24], but also to antibiotics belonging to a different class [Reference Colom25]. This concept is particularly true for P. aeruginosa as a pathogen harbouring multiple mechanisms of resistance [Reference Livermore26]. Moreover, antibiotic pressure could play a lesser role because horizontal transmission would be the main mechanism of acquisition in some cases [Reference Paterson27].
Concern has also been expressed about the promotion of infection by corticosteroids: recent studies have reported that corticotherapy is an independent predictor of nosocomial pneumonia sustained by Gram-negative pathogens and infection with antibiotic-resistant Gram-negative rods [Reference Raymond28, Reference Tejada Artigas29].
The presence of CVC was confirmed in our analysis as an important risk factor for P. aeruginosa BSI [Reference Schechner7, Reference Cheong30]. Interestingly, our study is the first to separately analyse the impact of this variable in MDR and non-MDR infections, demonstrating that it increases the risk of MDR P. aeruginosa BSI by almost 18 times.
In addition to the presence of CVC, the other risk factors associated with non-MDR P. aeruginosa BSI in our study were previous bacteraemia, neutropenia, and presence of urinary catheter.
To our knowledge, none of previous studies have reported an independent association between prior bacteraemia and P. aeruginosa BSI, this might be a marker for several other variables (such as previous use of antimicrobials, severe disease, invasive procedures), and not a risk factor by itself.
P. aeruginosa is one of the most common aetiological agents of Gram-negative bacteraemia in neutropenic patients, in particular in those with haematological malignancies [Reference Wisplinghoff31, Reference Tumbarello32]; in addition, neutropenia has been found to be independently associated to community-onset P. aeruginosa BSI by other authors [Reference Cheong30].
Regarding urinary catheterization, as mentioned above, Schechner et al. demonstrated that the presence of a urinary device was an independent predictor of P. aeruginosa bacteraemia upon hospital admission [Reference Schechner7]. All of these three factors were not found to be related to the onset of BSI caused by MDR P. aeruginosa in our study.
The all-cause 21-day mortality of 34% in patients with monomicrobial P. aeruginosa bacteraemia found here is in line with previous observations [Reference Osih3, Reference Aloush8]. In addition, we highlight the high mortality associated with MDR P. aeruginosa BSIs. Twenty-one days after infection onset, 50% of the patients with MDR P. aeruginosa infections had died, whereas non-MDR P. aeruginosa mortality was 24·2%.
The first prognostic indicator of an unfavourable clinical outcome is presentation with septic shock, which is an obvious result because the mortality for septic shock is usually more than double the mortality for severe sepsis, both in ICU and non-ICU patients [Reference Wenzel33].
Inadequate therapy emerged as another independent predictor of mortality in our patients. These findings are consistent with those of Lodise et al. [Reference Lodise2], who suggested that delaying appropriate therapy for approximately 2 days significantly increases the risk of mortality in patients with P. aeruginosa BSI. In addition, they found that antibiotic resistance in more than three classes was independently associated with a >52 h delay of appropriate therapy. Although the impact of appropriate empirical antimicrobial therapy for P. aeruginosa BSIs on patients' outcomes has not been clearly established, analogous findings have been reported by others [Reference Cheong34–Reference Tam36]. Nevertheless, some studies suggest that the use of appropriate initial empirical therapy is not critical to patients' outcomes [Reference Osih3, Reference Wu, Lin and Chang37].
Multivariate analysis revealed that isolation of a MDR strain of P. aeruginosa is an independent predictor of mortality in P. aeruginosa BSIs. Although a recent review showed high mortality rates in association with infections caused by antibiotic-resistant Gram-negative bacteria [Reference Giske5], the causal link between antibiotic resistance and poor outcome remains unclear, and inadequate therapy, which is more likely to occur in BSI caused by antibiotic-resistant bacteria, could be hypothesized. However, previous studies have reported significantly higher rates of mortality in patients with infections caused by antimicrobial-resistant Gram-negative rods [Reference Aloush8, Reference Tumbarello16, Reference Kang38]; although Blot et al. [Reference Blot39] reported no association between antimicrobial resistance and poor outcome in critically ill patients with nosocomial bacteremia caused by Gram-negative bacteria. In addition, Tam and colleagues [Reference Tam36] have recently reported that isolation of a MDR strain of P. aeruginosa was an independent predictor of mortality in P. aeruginosa BSIs.
Our study has certain limitations that must be acknowledged. First, the clonality of the isolates was not investigated (so that possible outbreaks could not be ruled out); in addition, the mechanisms of resistance were not analysed, and risk factors and prognosis might be different. Finally, some episodes could have been misclassified as having an unknown source, because a microbiological confirmation of a clinically suspected source was not requested.
In conclusion, decisions regarding the empirical treatment of P. aeruginosa BSIs must be based on sound knowledge of the local distribution of pathogens and their susceptibility patterns. In settings similar to ours, where MDR P. aeruginosa is fairly common, it is not easy to suggest empirical treatment of nosocomial BSIs due to the difficulty of ideally including drugs effective against these pathogens; in addition, preventive strategies (such as antibiotic policy, infection control measures, etc.) should be implemented in order to reduce the spread of these bacterial isolates. However, in patients at high risk for MDR P. aeruginosa BSI, empirical therapy including more than one anti-pseudomonal agent or a drug with high activity against the most resistant P. aeruginosa isolates (i.e. colistin) could be considered until susceptibility results become known.
ACKNOWLEDGEMENTS
This work was supported by a grant from the Università Cattolica del S. Cuore (Fondi Ateneo Linea D1-2008), Italy.
DECLARATION OF INTEREST
None.







