INTRODUCTION
Diarrhoea remains one of the leading causes of childhood morbidity and mortality, mainly in children aged <5 years living in developing countries. It is estimated that globally, 1·236 million children die because of diarrhoea each year, representing 15% of the total of <5-year-old child mortality [1]. Death due to diarrhoea has decreased significantly from 13·6/1000 to 4·9/1000 over the past three decades [Reference Rosenberg2]. However, the incidence of diarrhoea in children aged <5 years in developing countries is still high, with an average of 3·2 episodes per child per year [Reference Kosek, Bern and Guerrant3]. In Egypt, the diarrhoea incidence of 5·5 episodes per child per year is higher than the global average [Reference Rao4]. Many epidemiological studies have identified risk factors that have lead to low cost, simple and practical intervention measures to decrease the burden of diarrhoea; an intensive hand hygiene campaign for children in elementary schools in Cairo decreased the absence rate from school by 30% compared to controls. Another water quality intervention study in Bolivia showed that point-of-use water disinfection, safe water storage and community education decreased diarrhoea episodes by 44% in an intervention group compared to controls [Reference Sobel5–Reference Talaat11]. Sanitation and improved water supply are major and costly infrastructural changes that are needed for the prevention of diarrhoea, but these are long-term solutions at best for most of the world, and thus efforts are needed to identify and implement low-cost solutions [Reference Cairncross12]. Diarrhoea risk factors vary across populations according to geographical distribution and cultural practices, age and pathogens detected [Reference Sobel5, Reference Gascon13]. In this case-control study we aimed to identify modifiable risk factors associated with diarrhoea in children sged <5 years seeking treatment for diarrhoea in outpatient clinics at two university children's hospitals in Cairo, Egypt.
SUBJECTS AND METHODS
The study population was recruited from October 2007 to October 2009 from two children's hospitals located in two different universities which are referral hospitals that receive many diarrhoeal cases as well as other illnesses. Cairo University Children's Hospital serves most of Cairo and many other neighboring urban and rural areas, while Ain Shams University Children's Hospital serves East Cairo and neighbouring communities.
Cases were children aged <5 years seeking treatment for acute diarrhoea (i.e. lasting <14 days) at the outpatient clinics of the hospitals, from Saturday to Thursday, 09:00 to 14:00 hours. Diarrhoea was defined as the passage of ⩾3 loose or liquid stools in a 24-h period, or two loose or liquid stools in a 24-h period in addition to one or more associated symptom(s)/signs, including fever (oral temperature ⩾38·2°C, dysentery (gross blood in diarrhoeal stool) or abdominal pain/cramps temporally related to the diarrhoeal episode.
A control was selected for each case by selecting the next child in the clinic's admission registry from children presenting to the clinic with a chief complaint other than acute diarrhoeal illness, who had not experienced acute diarrhoeal illness during the preceding 30 days and did not present with a fever of unknown origin. Controls were identified and enrolled within 48 h of the matched case. The controls were matched for age and gender (6 months interval) with cases. If a potential control declined to participate, the next patient meeting the criteria for enrolment was approached regarding study entry.
After obtaining written informed consent, a paediatrician and a social worker interviewed the parents and/or guardians of both cases and controls. The questionnaire included information on family demographics such as: age, date of birth, gender, residence, date of hospitalization and questions about exposures related to the period before the onset of diarrhoea (or acute illness for control subjects). Other exposures included daycare situation, breastfeeding, use and handling of baby bottles and their contents, and consumption of foods and drinks with documentation of manner of preparation and storage. Queries related to household conditions included the number of people and rooms in the household and the presence of household members with diarrhoea before the onset of the child's illness. Queries related to hygiene included availability of running water and taps, water storage and handling practices, type of toilet and the availability of handwashing facilities. Also assessed were the caregiver's handwashing practices and the presence or absence of animals within and outside the house. Finally, the questionnaire measured socioeconomic indicators, such as parental education, occupation and ownership of the house and its contents.
Two rectal swabs and a stool specimen were collected from every case and control. One swab was placed in a clear plastic tube containing Cary–Blair transport medium, and a second was placed in a clear plastic tube containing Campy Thio Broth (CTB) transport medium. The rectal swabs and stool specimens were stored in a refrigerator at both sites and transferred in a cool box to United States Naval Medical Research Unit No.3 (NAMRU-3) no more than 3 days from collection. All the samples were processed at NAMRU-3, Cairo.
Upon arrival at NAMRU-3 the faecal swab samples were cultured for routine enteric pathogens using standard methods for Campylobacter, Salmonella, and Shigella. Additional characterizations of isolated Escherichia coli-like colonies were tested for enterotoxigenic E. coli (ETEC). ETEC colonies producing toxin were further tested for colonization factors using an immunodot-blot assay [Reference Svennerholm and Wiklund14]. Stool aliquots were screened for the presence of rotavirus and Cryptosporidium by commercial enzyme-linked immunosorbent assay kits (Premier Rotaclone®, Meridian Bioscience, USA and Techlab, USA) according to the manufacturers’ instructions.
Statistical analysis
The primary outcome variable was increased risk of acute diarrhoeal illness as measured by odds of exposure in cases compared to odds of exposure in controls. In addition to predictor variables such as family demographics and socioeconomic indicators, risk behaviour practices focusing specifically on drinking water treatment and storage, food preparation and storage, childcare, and hygiene practices of the caregiver were also examined. Univariate analyses were performed for all categorical variables and the strength of association between the behavioural factors and diarrhoea were estimated by calculation of odds ratios (OR) with 95% confidence intervals (CI). The McNemar test was used to determine the statistical difference in categorical variables between matched cases and controls and paired t test or Wilcoxon's matched-pairs signed-rank test were used for parametric and non-parametric testing, respectively, of continuous variables depending on normality assumptions. Variables with P < 0·2 in the univariate analysis were included in a multivariable conditional logistic regression analysis. Variables that were not significant in the univariate analysis were eliminated in an effort to include the maximum number of subjects in the final regression modelling. Interactions between primary exposure and other predictor variables were considered. All statistical analyses were performed with SAS version 9.1 (SAS Institute Inc., USA).
The variable of standard of living was used as a surrogate for socioeconomic status (SES). It was calculated based on assigning one point to any ownership of an asset that the head of household possessed (as assessed in the SES section of the questionnaire), such as a car, television, refrigerator, etc. The total score for each household was determined and the median for all scores was set as the cut-off value. Accordingly, those with a household value above that cut-off value were considered to have a high standard of living and below that value were considered to have a low standard of living.
Sample size was estimated from a range of risk-factor prevalence estimates previously published [Reference Sobel5]. Taking the estimates and logistical factors into consideration, the appropriate sample size was 400 cases and 400 controls from all study sites.
RESULTS
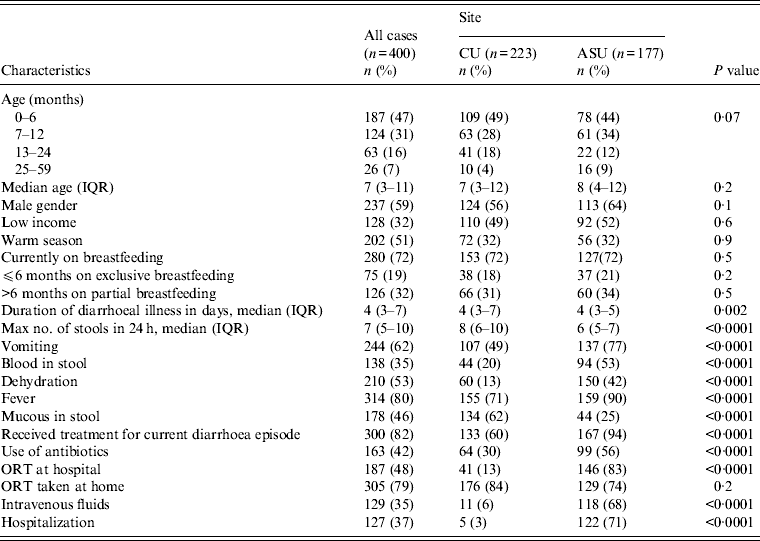
As the study was minimal risk, after initial screening the response rate in eligible cases was high and moderate in controls. The main reason for non-participation of cases was lack of interest in the study while the time required waiting until the child provided a stool sample was the main reason for controls refusing to participate. There were 400 cases and 400 controls enrolled in the study. Forty-seven per cent of cases were aged 0–6 months, 31% were aged 7–12 months, 16% were aged 13–24 months, and 7% were aged 25–59 months. Cairo University Children's Hospital had 223 pairs of cases and controls enrolled with 177 pairs from Ain Shams University Children's Hospital. Males constituted 59% of the participants with 51% of hospital visits during the summer season, from May to October (Table 1).
Table 1. Demographic and clinical characteristics of children seeking medical care for diarrhoea (cases) at Cairo University (CU) and Ain Shams University (ASU) paediatric hospitals, Cairo, Egypt, 2004–2009

IQR, Interquartile range; ORT, oral rehydration therapy.
There were no significant differences between cases enrolled from the two sites with regards to age (P = 0·2), gender (P = 0·1), SES (P = 0·6) or season of enrolment (P = 0·9). However, there were differences regarding the clinical presentation of diarrhoeal disease. Cases from Ain Shams University Children's Hospital were more likely to be dehydrated, have blood in stool, vomiting and fever (P = 0·0001), and were more likely to be hospitalized (P = 0·0001) than the Cairo University Children's Hospital cases (Table 1).
Cases presenting clinically with diarrhoea were also associated with fever (80%), vomiting (62%), blood in stool (35%), and dehydration (53%). Median duration of illness was 4 days [interquartile range (IQR) 3–7 days] and the maximum number of loose stools was seven per day (IQR 5–10). Since the start of the latest diarrhoea episode, 82% received treatment for the current diarrhoea episode including 42% who received antibiotics. Of all enrolled cases, 37% were hospitalized; 35% received intravenous fluids, and 48% received oral rehydration therapy (ORT) at the hospital. Additionally, 79% were prescribed ORT to be used at home (Table 1).
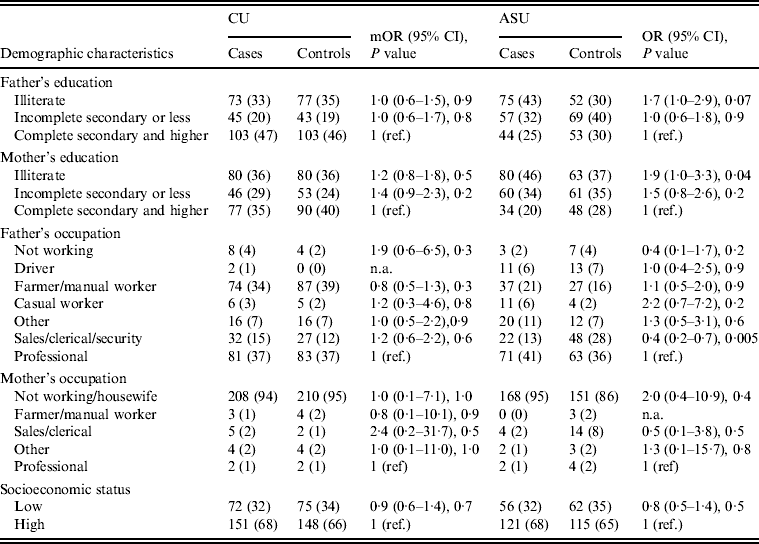
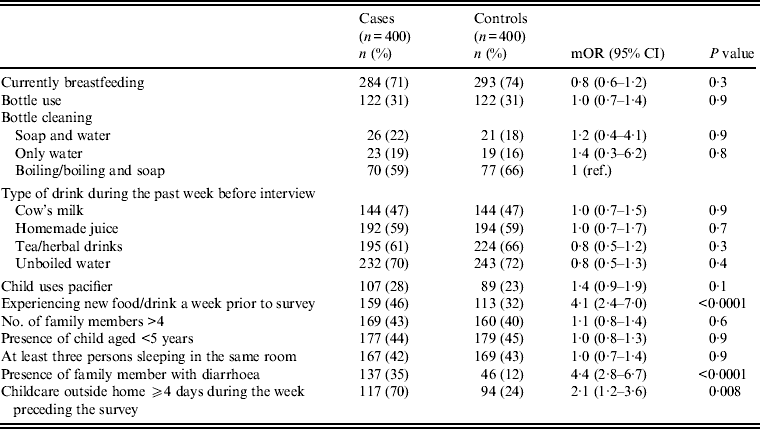
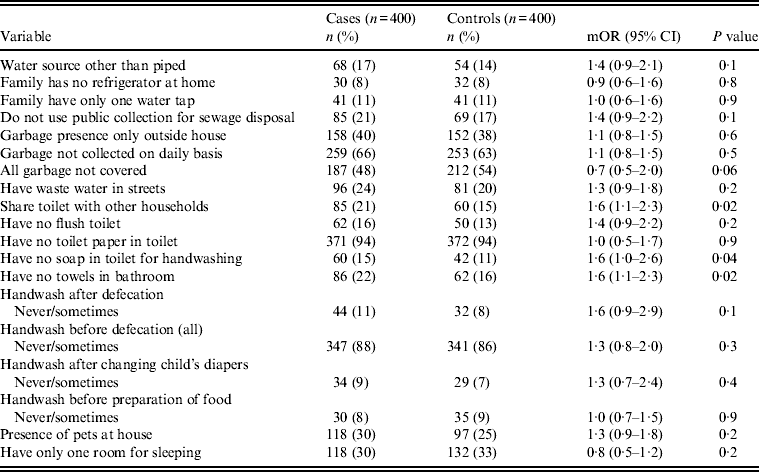
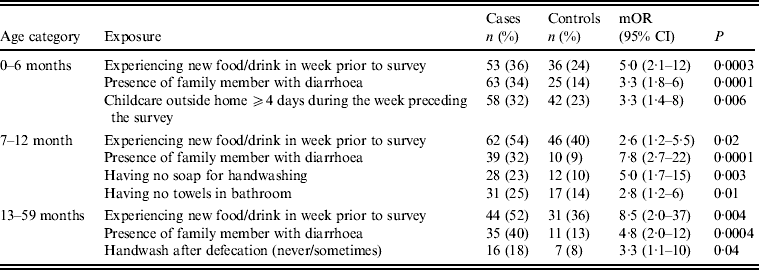
Univariate analysis (Tables 2–4) indicated several risk factors that were significantly associated with diarrhoeal illness including the presence of a family member with diarrhoea within 2 weeks before the child's diarrhoea began (OR 4·4, 95% CI 2·8–6·7, P < 0·0001), introduction of a new food that had never been eaten in the 7 days prior to the interview (OR 4·1, 95% CI 2·4–7) and childcare outside the home on any day during the 7 days preceding the survey (OR 2·1, 95% CI 1·2–3·6). Of household characteristics, three characteristics appeared more frequently in cases than controls. Cases (21%) used shared bathrooms, more often than controls (15%) (OR 1·6, 95% CI 1·1–2·3). Additionally, 15% of cases had no soap available in the bathroom compared to 11% of controls (OR 1·6, 95% CI 1·0–2·6), and 22% of cases had no towel available in the bathroom compared to 16% of controls (OR 1·6, 95% CI 1·1–2·3).
Table 2. Demographic characteristics suggested to be associated with diarrhoeal illness in matched pairs of children aged <5 years stratified by sites [Cairo University (CU) and Ain Shams University (ASU) paediatric hospitals], Cairo, Egypt, 2004–2009

mOR, Matched odds ratio; CI, confidence interval; ref. reference category; n.a., not available.
Table 3. Feeding characteristics, number of children and family members within household suggested to be associated with diarrhoeal illness in matched pairs of children aged <5 years seeking medical care at Cairo University (CU) and Ain Shams University (ASU) paediatric hospitals, Cairo, Egypt, 2004–2009

mOR, Matched odds ratio; CI, confidence interval; ref. reference category.
Table 4. Comparison between cases and controls according to risk factors related to house conditions of children aged <5 years with (cases) and without (controls) diarrhoea seeking medical care at Cairo University and Ain Shams University paediatric hospitals, Cairo, Egypt, 2004–2009

mOR, Matched odds ratio; CI, confidence interval.
Differences in demographic characteristics (paternal education and occupation, maternal education and occupation, and SES) between cases and controls were not significant (P < 0·05).
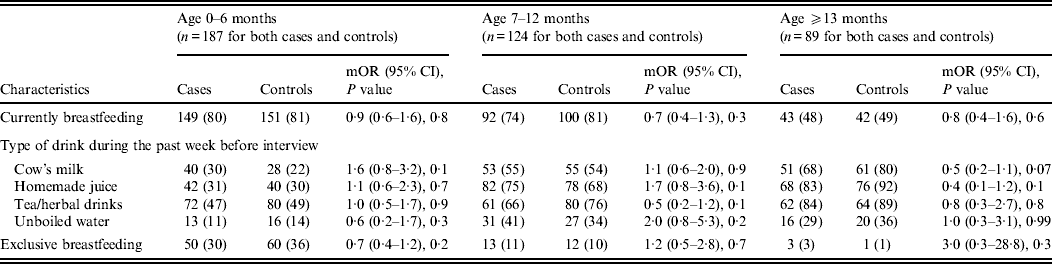
Other feeding and hygiene practices also showed no statistical difference between cases and controls. These included breastfeeding status, bottle use and bottle cleaning practices, type of drink consumed during the week before the interview (i.e. cow's milk, homemade juice, tea, herbal drink, unboiled water), pacifier use, more than four family members living in the same household, presence of another child aged <5 years at home, and at least three persons sleeping in the same room (Table 3 and 5).
Table 5. Feeding characteristics stratified by age of children aged <5 years with (cases) and without (controls) diarrhoea seeking medical care at Cairo University and Ain Shams University paediatric hospitals, Cairo, Egypt, 2004–2009

mOR, Matched odds ratio; CI, confidence interval.
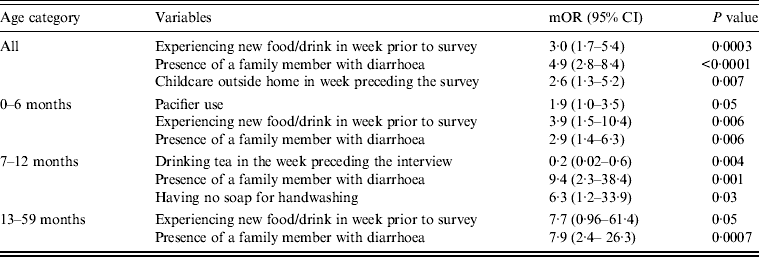
Upon stratifying by age (0–6, 7–12, ⩾13 months) (Table 2), univariate analyses showed that risk factors associated with diarrhoeal illness for the 0–6 months age group included the presence of a family member with diarrhoea within 2 weeks before the child's diarrhoea began (OR 3·3, 95% CI 1·8–5·8), introduction of new food not eaten in the 7 days prior to the interview (OR 5·0, 95% CI 2·1–12) and childcare outside the home on any day during the 7 days preceding the survey (OR 3·3, 95% CI 1·4–7·7) (Table 6).
Table 6. Risk and protective factors significantly associated with diarrhoeal illness in matched cases and controls aged <5 years seeking medical care at Cairo University and Ain Shams University paediatric hospitals, Cairo, Egypt, 2004–2009

mOR, Matched odds ratio; CI, confidence interval.
For the 7–12 months age group, risk factors were: the presence of a family member with diarrhoea within 2 weeks before the child's diarrhoea began (OR 7·8, 95% CI 2·7–22); the child began eating a food he/she had never eaten before in the 7 days prior to the interview (OR 2·6, 95% CI 1·2–5·5), no soap in the bathroom (OR5, 95% CI 1·7–14·6) and no towels in the bathroom (OR 2·8, 95% CI 1·2–6·2) (Table 6).
For the 1–5 years age group, risk factors were: the presence of a family member with diarrhoea within 2 weeks before the child's diarrhoea began (OR 4·8, 95% CI 2·0–11·6); the child having eaten a food not previously eaten in the 7 days prior to the interview (OR 8·5, 95% CI 2·0–36·8), lack of caregiver handwashing after cleaning the child after defecation (OR 3·3, 95% CI 1·1–10) and the presence of pets at home (OR 2·1, 95% CI 1·0–4·3) (Table 6).
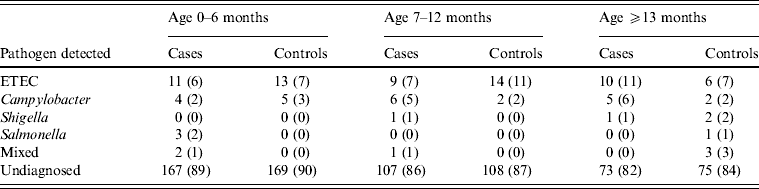
An identified pathogen was isolated from 34% (n = 134) of cases vs. 13% (n = 52) of controls. A bacterial pathogen was isolated from 13% of diarrhoea stool samples vs. 12% of controls, with no significant difference between them. ETEC and Campylobacter spp. were isolated from 8% and 4% of cases vs. 9% and 2% of controls respectively, while Shigella spp., Salmonella spp. and mixed infections were ⩽1% for both cases and controls. On the other hand, rotavirus, the only enteric virus tested for, was detected in 20% of cases vs. 13% of the 30 stool samples tested from controls. Additionally, Cryptosporidium was detected in 5% of cases vs. 3% of the 29 stool samples tested from controls (Table 7).
Table 7. Distribution of pathogens stratified by age detected in cases and controls seeking medical care at Cairo University and Ain Shams University paediatric hospitals, Cairo, Egypt, 2004–2009

ETEC, Enterotoxigenic Escherichia coli.
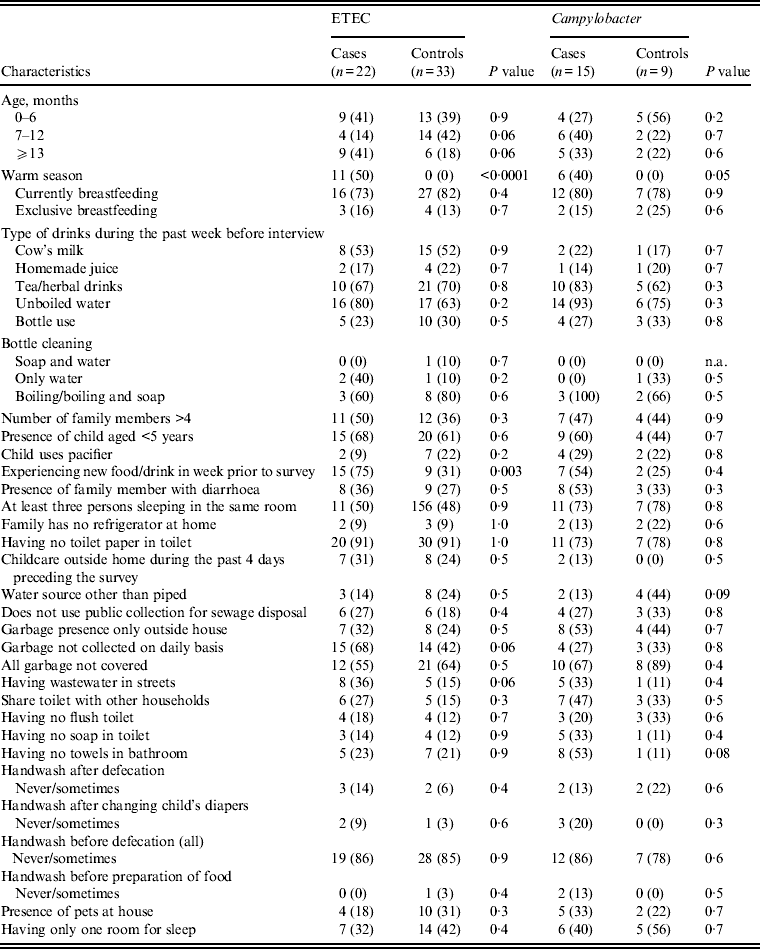
Univariate analysis of suggested risk factors associated with diarrhoea and mainly ETEC and Campylobacter spp. were performed (Table 8). ETEC was significantly associated with diarrhoea during the warm season and children experiencing new food in the 7 days preceding the interview was also associated with ETEC diarrhoea. Additionally, children living in houses where garabage was not collected on daily basis and where the waste water was collected in the streets of their living area tended to have ETEC-associated diarrhoea (P = 0·06). For Campylobacter spp.-associated diarrhoea the risk increased in the warm season but was not associated with any other suggested risk factor.
Table 8. Pathogen distribution according to demographics and some selected risk factor in diarrhoea cases and their matched controls seeking medical care at Cairo University and Ain Shams University paediatric hospitals, Cairo, Egypt, 2004–2009

ETEC, Enterotoxigenic Escherichia coli.
Multivariate conditional logistic regression (Table 9) showed three independent risk factors for overall diarrhoea: if the child had eaten any food not previously eaten in the 7 days prior to the interview (OR 3, 95% CI 1·7–5·4), presence of a family member with diarrhoea (OR 4·9, 95% CI 2·8–8·4), and childcare outside the home for ⩾4 days during the week preceding the survey (OR 2·6, 95% CI 1·3–5·2). Attributable fractions for these risk factors were 0·16, 0·17 and 0·18, respectively.
Table 9. Predictors of diarrhoeal illness on multivariate analysis in children aged <5 years seeking medical care at Cairo University and Ain Shams University paediatric hospitals, Cairo, Egypt, 2004–2009

mOR, Matched odds ratio; CI, confidence interval.
Risk factors identified by multiple logistic regressions for the 0–6 months age group were: introduction of new food (OR 3·9, 95% CI 1·5–10·4), presence of a family member with diarrhoea (OR 2·9, 95% CI 1·4–6·3), and the child's use of a pacifier (OR 1·9, 95% CI 1·0–3·5). For the 7 months to <1 year age group, independent risk factors identified were: presence of a family member with diarrhoea (OR 9·4, 95% CI 2·3–38·4) and absence of soap in the bathroom (OR 6·3, 95% CI 1·2–33·9). However, there was one protective factor: consumption of tea and/or herbal drinks in the week preceding the interview (OR 0·2, 95% CI 0·02–0·6).
Finally, for the 1 to <5 years age group, identified risk factors were introduction to a new food (OR 7·7, 95% CI 1·0–61) and the presence of a family member with diarrhoea (OR 7·9, 95% CI 2·4–26·3).
DISCUSSION
A combination of environmental, microbiological, social and host immune statuses together determine the risk associated with the human burden of diarrhoea [Reference Sobel5, Reference Pruss-Ustun, Bonjour and Corvalan15]. Sanitation and safe/clean water supplies are needed for the prevention of diarrhoea within a society but are not sufficient unless paired with changes in modifiable risk behaviours. Protective measures like improved sanitation and establishing a microbiologically and chemically safe water supply can be implemented by governments, but these efforts require considerable time and effort and are costly. Alternatively, modifying risk behaviours can also reduce the burden of diarrhoeal diseases to affected segments of the population, offering protection that is more readily implemented [Reference Quick10, Reference Talaat11].
Of the demographic epidemiological characteristics studied, the presence of a family member with diarrhoea had the highest impact on diarrhoea burden. A child who had a family member with diarrhoea was five times more likely to have diarrhoea compared to those without a family member with diarrhoea; additionally this risk factor had an attributable fraction of 0·17. This finding was also observed in other studies [Reference Sobel5, Reference Blake16]. Although an unidentified shared exposure may have led to diarrhoea in household members, the effect of this risk factor on all age groups studied suggests a high rate of intrahousehold transmission and the need for specific measures to minimize this transmission. Many infectious agents are able to cause diarrhoeal disease through faecal–oral transmission and in small inocula, such as Shigella spp. Other microbes require larger inocula, but close contact with an infected person has led to the spread of other microbes such as enterohemorrhagic E. coli, Campylobacter spp., norovirus, rotavirus, Giardia lamblia, Cryptosporidium parvum, and Entamoeba histolytica [Reference Pawlowski, Warren and Guerrant17]. Pathogens detected in the current study were mainly observed in children aged <1 year, with rotavirus the most common pathogen detected. Blake et al., showed that the presence of a household member with diarrhoea preceding the case's illness was the risk factor with the highest OR for children aged <1 year [Reference Blake16]. This finding was further confirmed by Sobel et al., who added that the presence of an infected household member was strongly associated with rotavirus infection [Reference Sobel5]. Studies have shown that improvement in hygiene practice, sanitation and handwashing may effectively decrease diarrhoea morbidity in general [Reference Cairncross12, Reference Huttly, Morris and Pisani18].
Exposure of the child to a new food/drink in the 7 days prior to interview was also a risk factor for diarrhoea. The effect of the introduction of a new food was evident in all age groups in the primary analysis; however, in multivariate analysis the effect was evident only for age groups 0–6 months and 13 months to <5 years. In the 7–12 months age group there may not have been sufficient power to detect a significant difference. The 0–6 months and 13 months to <5 years age groups differed in feeding practices, mobility, and immunity; the mechanism of how a new food is introduced in these two age groups is likely to be different. The impact of the introduction of a new food was higher in children aged >1 year (OR 7·7) compared to children agred <6 months (OR 4). This finding may be linked to the observation that in general, children aged >1 year start to experience a greater variety of food types compared to younger children. However, for the 7–12 months age group, drinking herbal tea in the 7 days prior to interview may be a protective factor against diarrhoea (OR 0·2). Along the lines of this observation, several studies have shown that specific types of food may increase or decrease the burden of diarrhoea. One study from Uganda showed that consumption of raw chicken eggs was significantly (P < 0·01) and strongly (OR 99) associated with diarrhoea in residents of Kampala district [Reference Nasinyama19]. Consumption of homemade fruit juice was associated with diarrhoea in a study from Brazil [Reference Sobel5] and in another study from Mexico, asymptomatic heat labile-ETEC infection increased 400% in children who received oat gruel (hazard rate 4·01, 95% CI 2·77–5·24), while drinking a herbal tea reduced the hazard of symptomatic infection by 90% [Reference Long20]. Breastfeeding was not protective against diarrhoea in our study, similar to other studies [Reference Sobel5, Reference Long20], but contrasting with other studies that have shown breastfeeding to be protective during infancy [Reference Quiroga21–Reference Dennehy23]. Breastfeeding has also been reported to have limited protection against rotavirus diarrhoea after the first months of infancy [Reference Glass24]. The reason why breastfeeding was not protective for individual pathogens causing diarrhoea in our study was not clear. However, possible reasons include: pathogen detection was limited to only a few pathogens which may not be protected by breastfeeding, perhaps a significant number of children used antibiotics before sample collection and/or a limited number of samples were collected from controls for viral and parasitic isolation. Studies have shown that consumption of contaminated food, mainly in the weaning period, is associated with diarrhoea. Thus, improvement of food safety through community-based education programmes may be an effective tool to decrease the burden of diarrhoea in this population [Reference Sheth and Dwivedi25, Reference Sheth and Obrah26].
Another hygiene practice identified as an independent risk factor for diarrhoea in the 0–6 months age group was pacifier use. Infants who used pacifiers were twice as likely to have diarrhoea than controls. Other studies have reported the same finding from developed and developing countries [Reference Victora27–Reference North, Fleming and Golding29]. In one study, use of pacifiers by infants aged <6 months not only increased the risk for diarrhoea but also increased risk of early weaning [Reference Victora27]. In another study, pacifiers were linked not only to diarrhoea, but also to respiratory illnesses [Reference North, Fleming and Golding29].
A related hygiene variable that was recognized as an independent risk factor for diarrhoea was childcare outside the home (daycare) for ⩾4 days in the 7 days prior to the survey (OR 2·6). Association of daycare with diarrhoea has been reported in many studies from developed and developing countries; in developing countries, studies have shown that care of the child at home during the week preceding the illness was protective and that informal daycare in private homes was a significant risk factor for diarrhoeal illness [Reference Sobel5, Reference Blake16, Reference Molbak30]. In developed countries, studies have shown that any household daycare is safer than formal daycare [Reference Sobel5, Reference Bartlett31, Reference Louhiala32]. Other studies from developing countries have indicated that children attending daycare centres may be exposed to diarrhoeal epidemics [Reference Isakbaeva33, Reference Rosenfeldt34]. Additionally, improving hygiene practices was found to be the only intervention in the childcare centre environment that had good potential to reduce diarrhoea in attendees [Reference Barros35].
Absence of soap in the bathroom for handwashing was a further risk factor for diarrhoea for children aged 7–12 months (OR 6·3). Association between poor hygienic conditions and diarrhoea has been reported in many studies [Reference Knight7, Reference Bhattacharya36]. One study reported that the most immediate preventive impact can be achieved by promoting handwashing with soap [Reference Taylor and Greenough37]. Another study reported that handwashing with soap decreased diarrhoea by 48% [Reference Cairncross12]. On the other hand, one study reported that handwashing itself either with or without soap after eating, defecation, or cleaning the child can reduce subsequent diarrhoea [Reference Luby38].
Limitations of our study include recall bias of the caregivers, which may have had an effect on the study results. However, we attempted to limit the time of recall to a narrow period (1–2 weeks prior to the survey). The stratification of cases and controls by ages (three age categories) may also have influenced the risk factors detected for each age group, due in part to the smaller population in each stratum. Additionally, this may have decreased the power because the study was conducted in outpatient clinics, the number of stool samples collected from the controls was low, potentially affecting the detection of viral and parasitic pathogens in controls, and possibly affecting the ability to detect pathogen-specific risk factors.
In summary, this study provides useful data which needs further exploration. While we found that the presence of another household member with diarrhoea, introduction to new foods, and having childcare outside the home increased risk, these are not in and of themselves easily modifiable. They do suggest that further study is needed to evaluate what modifiable behaviours such as handwashing, increased peri-domestic sanitation, and food safety practices in the home might have on reduction of disease in these populations. Given that major improvements in sanitation, access to clean water, and vaccines to prevent diarrhoea are decades away from availability, all that can be done, should be done now to decrease the burden of diarrhoeal diseases worldwide.
ACKNOWLEDGMENTS
We acknowledge the contribution of Dr Yassmin Gamal El Gendy from Ain Shams University and Dr Ayman El Shamy from Cairo University for clinical support; members of data collection teams from both universities for field work support; Mrs Yassmin Farid and Mr Mohammed Fakhry for data entry; Mr Abd El Hakam, Mr Salem Ibrahim, Mr Gamal El Okla, Mr Salah Motaweai, Mr Ismail Raafat and Mrs Iman Touni for technical assistance. We gratefully acknowledge the extraordinary cooperation from the study participants and the kind support of the entire staff of the paediatric hospitals in both universities.
The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, the U.S. Government, nor the Egyptian Ministry of Health and Population. The work was funded by work unit #847705.82000.25GB.E0018 and #6000.RAD1.D.E0301. The study protocol NAMRU3.2006.0007 (IRB No.189) was approved by the U.S. Naval Medical Research Unit No. 3 Institutional Review Board in compliance with all applicable Federal regulations governing the protection of human subjects.
DECLARATION OF INTEREST
The authors had no potential, perceived, or real conflict of interest. The study sponsor had no involvement in (1) study design; (2) the collection, analysis, and interpretation of data; (3) the writing of the report; nor (4) the decision to submit the manuscript for publication.