INTRODUCTION
Microsporidia are considered to be emerging and opportunistic infections in humans. Enterocytozoon (Ent.) bieneusi and Encephalitozoon (E.) intestinalis are the most common microsporidia infecting humans that are associated with diarrhoea and systemic disease. Persistent or self-limiting diarrhoea are common symptoms associated with microsporidiosis in both immunodeficient or immunocompetent individuals. Microsporidial spores appear to be relatively resistant under environmental conditions. Species of microsporidia infecting humans and animals have been identified in water sources, raising concern about waterborne transmission. In the developing world, parasitic disease contributes heavily to the burden of diarrhoeal diseases. Patients, who are malnourished, transplant recipients and those with acquired immune deficiency syndrome (AIDS), are especially at risk of increased morbidity and mortality from microsporidiosis. Twenty to forty per cent of HIV-positive individuals in the developing world develop microsporidiosis [Reference Petry1–Reference Sax4].
Microsporidia are small (1–2 μm), single-celled, obligate intracellular parasites characterized by a polar filament that is used for invasion of the host cell [Reference Muller5]. Mature microsporidial spores have thick walls and can pass through water treatment filters and are resistant to chlorine at concentrations used to treat drinking water. Microsporidial spores have been found in drinking water sources, soil and domestic and wild animals, suggesting the possibility of waterborne, foodborne and zoonotic transmission. Microsporidia are on the Contaminant Candidate List because their transmission routes are unknown. Spore identification, removal and inactivation in drinking water are difficult and human infections are difficult to treat [6, Reference Didier7]. Microsporidial spores detected in a variety of surface waters are implicated as a source of human infection based on epidemiological data [Reference Fournier8–Reference DeGirolami13]. Infections with microsporidia in immunocompetent individuals such as travellers have also been described [Reference Muller5, Reference Weber14, Reference Weiss15].
This study was conducted to determine the incidence of E. intestinalis and Ent. bieneusi infection in our patients presenting with chronic diarrhoea and to compare them with healthy controls and those with hepatocellular carcinoma (HCC) superimposed on chronic liver disease associated with hepatitis C virus (HCV). In patients with HCC, impaired immunological competence and malnourishment are known to cause an increase in their susceptibility to infection [Reference Iida16]. The phagocytic and bactericidal activities of neutrophils and the percentage of natural killer cells are significantly reduced in patients with HCC [Reference Iida16]. Moreover, dysregulation of both type 1-related and type 2-related host immunity has been described in patients with HCV-associated HCC [Reference Suruki17].
MATERIALS AND METHODS
Subjects
The stool samples of 330 patients were examined. Of these, 171 patients had chronic diarrhoea with >3 stools a day for at least 4 weeks and <12 weeks. Individual controls (n=141) consisted of healthy members of paramedical staff without diarrhoea and 18 (5%) with HCC associated with chronic liver disease with hepatitis C. There were 222 (67%) males and 108 (33%) females with a mean age of 41±14 years (range 15–83 years). The patients attended the Gastroenterology Outpatient clinic at the Aga Khan University, Karachi between November 2008 and December 2010. The mean age of patients with chronic diarrhoea was 40±15 years (range 16–83) with a male:female ratio of 13:6. The male predominance reflects the tendency of male patients to present more commonly to consulting clinic with this complaint. In healthy controls, the mean age of patients was 42±14 years. All patients underwent history, physical examination, complete blood count, liver function test, serum creatinine, electrolytes, stool microscopy and polymerase chain reaction (PCR) for Ent. bieneusi and E. intestinalis. HIV status was negative in all patients and controls. A note was made of the presence of protozoa such as Blastocystis hominis, Giardia lamblia, Entamoeba, etc. on stool microscopic examination. HCC was diagnosed on the basis of α-fetoprotein level and ultrasound. Serology and PCR were performed for hepatitis B (HBV), HCV and delta (D) virus. All patients with HCC were positive for HCV. The study was approved by the institutional ethics review committee and was performed in accordance with the ethical standards laid down in the Declaration of Helsinki, 1964. All persons gave their informed consent prior to their inclusion in the study. All the stool specimens were processed by microscopy with modified trichrome staining and PCR. The diagnosis of microsporidial infection was made when both stool microscopy and PCR were positive. A microbiological investigation was also performed to detect Salmonella spp., Campylobacter jejuni, Clostridium difficile and Vibrio cholerae. However, a viral screen was not performed on stool specimens due to cost restrictions.
Microscopy of faecal smear
Faecal sample microscopy for demonstrating microsporidia was performed as described previously [6]. Wet smears in saline and iodine were examined under both low-power (×10) and high-power (×40–100) for the presence of parasites. Stool smears were fixed in ethanol and stained with modified trichrome stain in order to detect microsporidia [Reference Gamboa-Dominguez3].
Extraction of genomic DNA
Stool DNA was extracted by using Stool DNA Extraction kit (Qiagen, USA) according to the manufacturer's protocol. Extracted DNA was stored at −20°C until PCR was performed for microsporidia.
PCR
The primers V1 (5′-CACCAGGTTGTTCTGCCTGAC-3′) and EB450 (5′-ACTCAGGTGTTATACTCACGTC-3′) described by Zhu et al. [Reference Zhu18] were used to amplify Ent. bieneusi DNA. The primers V1 and SI500 (5′-CTCGCTCCTTTACACTCGAA-3′) described by Weiss et al. [Reference Weiss19] were used to amplify E. intestinalis DNA. The PCR reaction volume was 25 μl, comprising of 2·5 μl of 10× PCR buffer (Promega, USA), 2·0 μl of 25 mm MgCl2 (Promega), 0·4 μl dNTP mixture (10 mm of each dNTP, Promega), 0·5 μl (5 IU/μl) of Taq polymerase (Promega), 1 μl (0·25 μm) primers (IDT) and 2·0 μl of template DNA.
For the two sets of primers, one amplification cycle consisted of an initial denaturation of target DNA at 94°C for 10 min, followed by denaturation at 94°C for 1 min, primer annealing at 55°C for 2 min, and elongation at 72°C for 3 min. The last elongation step was extended to 10 min. Samples were amplified through 35 consecutive cycles. The PCR products and molecular markers were electrophoresed in 2% agarose gel with Tris-acetate-EDTA electrophoresis buffer. The size markers were 100-bp ladder (Promega). The PCR amplification was repeated at least three times. Bands were visualized by the imaging system (Gel Doc 2000, Gel Documentation System, Bio-Rad, UK).
Sequence analysis of small subunit unit ribosomal RNA and BLAST query
The DNA fragments amplified by E. intestinalis PCRs were purified by Qiagen quick PCR purification kit (Qiagen) and sequenced using both the forward and reverse primers (Table 1) to verify that they represented the E. intestinalis small subunit unit ribosomal RNA gene. Sequence analysis was performed by Macrogen (South Korea). Sequence comparison was carried out using the Blast program and the GenBank database.
Table 1. Characteristics of patients in different groups (N=330)
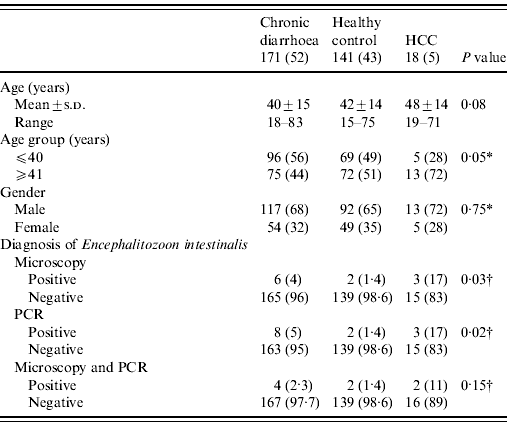
HCC, Hepatocellular carcinoma; PCR, polymerase chain reaction.
Values given are n (%) unless stated otherwise.
A P value of <0·05 was considered as statistically significant.
* Comparison between groups assessed using the χ2 test.
† Comparison between groups assessed using the likelihood ratio test.
Statistical method
Results are expressed as mean±standard deviation for continuous variables (e.g. age) and number (percentage) for categorical data (e.g. gender, stool culture, diarrhoea, etc.). Univariate analysis was performed by using the independent sample t test, Pearson χ2 test and Fisher's exact test where appropriate. Comparison between groups was assessed using the χ2 test, Fisher's exact test or likelihood ratio test, as appropriate. The concordance between stool microscopy and PCR was determined by the kappa (κ) test. A P value of <0·05 was considered statistically significant. All P values were two-sided. Statistical interpretation of data was performed by using the computerized software program SPSS version 19.0 (SPSS Inc., USA).
RESULTS
Symptoms
A total of 193/330 (58%) stool samples were submitted from patients with diarrhoea while 171/330 (52%) had abdominal discomfort or pain.
Stool microscopy
Stool microscopy revealed B. hominis in 110/330 (33%), Entamoeba dispar in 53/330 (16%), G. lamblia in 19/330 (6%), Entamoeba histolytica in 18/330 (6%) and microsporidia in 11/330 (3%), respectively. Microsporidia was also found by microscopy with trichrome staining in 11/330 (3%) and PCR for E. intestinalis in 13/330 (4%) (Table 1). There were no cases of microsporidia that were found by microscopy and not detected by PCR. Eight of 330 (2·4%) had microsporidia detected by both microscopy and PCR (Table 1). Only 4/18 (22%) with HCC had symptoms of diarrhoea.
Diagnostic yield of various methods for microsporidia
Microscopy with staining detected microsporidia in 6/171 (4%) stool specimens from patients with chronic diarrhoea, 2/141 (1·4%) stool specimens from controls and in 3/18 (17%) stool specimens from patients with HCC (Table 1). PCR of E. intestinalis was positive in 8/171 (5%) cases with chronic diarrhoea, 2/141 (1·4%) in controls and in 3/18 (17%) with HCC (Table 1) while Ent. bieneusi was negative. The correlation between microscopy with staining and PCR for E. intestinalis was 8 (73%) (κ=0·654, P<0·001).
Comparison of distribution of E. intestinalis in different groups
Four of 171 (2·3%) stool specimens from patients with chronic diarrhoea, 2/141 (1·4%) from controls and 2/18 (11%) with HCC had E. intestinalis infection (Table 1). In stool specimens from patients with chronic diarrhoea, E. intestinalis was positive in 4/171 (2·3%) compared to 2/141 (1·4%) in stool specimens from controls (P=0·69) (Table 2). In stool specimens from patients with HCC, 2/18 (11%) were positive for E. intestinalis compared to 2/141 (1·4%) in controls (P=0·06) (Table 2).
Table 2. Comparison of different groups with both microscopy and PCR for microsporidia

HCC, Hepatocellular carcinoma; PCR, polymerase chain reaction.
Values given are n (%).
A P value of <0·05 was considered as statistically significant.
* Comparison between groups was assessed using Fisher's exact test.
Distribution of mixed infection in different groups
In stool specimens from patients with chronic diarrhoea 5/171(3%) (P<0·001) and 3/18 (17%) (P=0·03) patients with HCC demonstrated E. intestinalis compared to 2/141(1%) healthy controls, respectively (Table 3). Co-infection of E. intestinalis infection was present with B. hominis in 2/171 (1%) and in 1/171 (1%) with both B. hominis and Entamoeba dispar in chronic diarrhoea (Table 3).
Table 3. Distribution of protozoa parasite in different groups
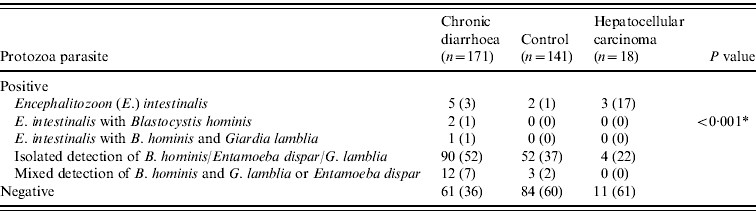
Values given are n (%).
* Comparison between groups was assessed using the likelihood ratio.
A P value of <0·05 was considered as statistically significant.
Sequence analysis of small subunit unit ribosomal RNA and BLAST query
The DNA fragments amplified by PCR were purified by Qiagen quick PCR purification kit (Qiagen) and sequenced using both the forward and reverse primers V1 and SI520 to verify that they represented E. intestinalis small subunit unit ribosomal RNA gene with GenBank accession numbers JF932504, JF932505, JF932506 and JF932507. Homology of the DNA sequences to published sequences was determined using the BLAST window on the National Centre for Biotechnology Information (NCBI) site at http://www.ncbi.nlm.nih.gov/BLAST/. PCR product sequences aligned well with the sequences of E. intestinalis CP001951.1, CP001946.1, CP001945.1, CP001942.1 and DQ453122.1.
DISCUSSION
In non-immunocompromised patients, both asymptomatic and symptomatic microsporidial infections have been described, which are usually seen in travellers or residents of tropical areas. The majority of symptomatic infections in humans occur in patients with HIV infection who are significantly immunocompromised. The elderly, with a mean age 75 years were identified as at risk of Ent. bieneusi infection when presenting with chronic diarrhoea [Reference van Gool20]. Case reports have also documented travellers' diarrhoea caused by Ent. bieneusi in normal hosts [Reference Lopez-Velez12, Reference Lores21, Reference Fournier22]. E. intestinalis has been found in stool samples of travellers' with chronic diarrhea [Reference Bryan, Weber and Schwartz23].
This study highlights E. intestinalis as the cause of chronic diarrhoea among other more common parasites such as G. lamblia, Entamoeba histolytica, etc. The point prevalence of microsporidial infection in patients with chronic diarrhoea was rather low (4% for microscopy, 5% for PCR, and 2·3% for both microscopy and PCR) and not much different from controls (i.e. 1·4% for both microscopy and PCR). E. intestinalis was shown in patients with HCC, chronic diarrhoea and in controls. There was a relatively high percentage of microsporidial infection in HCC patients. However, the number of patients was too small in this group to make a meaningful comparison with the other groups of chronic diarrhoea and controls. Moreover, the lack of follow-up does not allow an assessment of the significance and duration of microsporidial infection in this group. Co-infection of E. intestinalis with B. hominis was demonstrated in two cases and with B. hominis and Entamoeba dispar in one case with chronic diarrhoea. However, none of the cases with HCC described here had E. intestinalis infection combined with any of the other parasites. There were only isolated infections with B. hominis, Entamoeba dispar or G. lamblia (Table 3). Testing with modified trichrome staining and PCR for E. intestinalis demonstrated a high degree of concordance. These parasites are easily detected by light microscopy when infections are heavy. However, early infections without spores, or light infections with low numbers of spores, can easily be missed. E. intestinalis was detected in 13 cases by PCR and in only 11 by modified trichrome staining. It was probably missed by staining in cases where spores were low in numbers. It is surprising that E. intestinalis was identified while Ent. bieneusi was not detected in any group in this study. This could be explained by the lack of exposure to the environmental sources of Ent. bieneusi such as pigs, cats and cattle, while E. intestinalis occur in goats and donkeys which are common in urban areas of Pakistan, being kept as pets or for pulling carts for transportation of goods [Reference Dengjel24, Reference Bornay-Llinares25]. The presence of E. intestinalis has also been confirmed in tertiary sewage effluent, surface water and groundwater [Reference Dowd, Gerba and Pepper26]. Locally, water used for domestic purposes is usually supplied by the underground city municipal pipeline which is in close proximity to the sewage pipeline. Over the years with wear and tear they have been known to break down causing undetected contamination of domestic water supply by sewage. Moreover, in studies from neighbouring India, the prevalence of intestinal parasitic infections in HIV-positive patients with diarrhoea reported Ent. bieneusi in only 2·5% [Reference Mohandas, Sud and Malla27] while Cryptosporidium parvum and G. lamblia were the most common infecting parasites in these patients [Reference Mohandas, Sud and Malla27, Reference Becker28].
This is the first report of microsporidial infection from Pakistan. It supports the need to include microsporidia in the differential diagnoses for causes of chronic diarrhoea in both HIV-infected [Reference Eeftinck Schattenkerk2] and non-HIV-infected groups of patients. Specific stains for microsporidia should be requested in particular cases since routine examination for ova and parasites does not usually detect microsporidia spores. Faecal leukocytes and blood are commonly absent since microsporidial infection is not associated with a significant inflammatory reaction. PCR of microsporidia can be used for quick detection of microsporidial infection [Reference Weber29]. Modified trichrome staining has a reported sensitivity and specificity of 100% and 83%, respectively [Reference Ignatius30]. In our study there was a significant concordance between PCR and modified Trichrome staining of the specimen. Available fluorescent techniques including Uvitex 2B (Ciba Geigy, UK), Calcofluor White M2R (Sigma, USA), the FungiFluor kit (Polysciences, USA) and Fungiqual A have similar sensitivity and specificity to modified trichrome staining [Reference DeGirolami13, Reference Mo and Drancourt31, Reference Sheoran32]. However, microscopy is known to be highly dependent on the expertise of the examiner. Monoclonal antibodies to Encephalitozoon spp. have been described as improving detection of microsporidia in clinical specimens [Reference Fedorko and Hijazi33, Reference Müller34]. Other techniques include serological assays (which detect IgM and IgG anti-microsporidial antibodies), tissue culture, and indirect immunofluorescence and PCR [Reference DeGirolami13, Reference Zhu18, Reference Franzen and Muller35, Reference Graczyk36]. Multiplex FISH assay has been described as more sensitive than both Chromotrope-2R and Calcofluor White M2R stains and it has been used for assessing spore-shedding intensity in intestinal microsporidiosis and identification of microsporidial spores [Reference Graczyk36]. In conclusion, microsporidial infection may present with chronic diarrhoea. An examination of stool specimen with modified trichrome staining or PCR for E. intestinalis should be performed for selected patients with intractable symptoms of diarrhoea.
ACKNOWLEDGEMENTS
This study was supported by the Higher Educational Commission Grant (ref. 20-774/R&D/06/267). We are grateful to the staff of the Juma Building Research Laboratory for their help during this study.
DECLARATION OF INTEREST
None.





