Alveolar echinococcosis (AE), caused by the larval stage of the small tapeworm Echinococcus multilocularis, is one of the most severe parasitic diseases in humans and can be fatal if left untreated. Humans are infected by accidentally ingesting the parasite's eggs that are shed into the environment via the faeces of infected definitive hosts, mainly the red fox in Europe, although domestic dogs can also play this role. Following infection, 5–15 years may pass before the disease becomes apparent. Until recently, AE had been reported in at least nine European countries [Reference Kern1]. Switzerland is one of these endemic countries and in the past decades the annual incidence of AE in Switzerland has increased 2·5-fold [Reference Schweiger2]. Preceding this event, the number of foxes hunted in Switzerland increased 4-fold, indicating a direct relationship between the number of infected foxes shedding eggs in the environment and the development of the disease in humans. In Western Europe, the fox population density appears to be increasing [Reference Chautan, Pontier and Artois3]. The number of human cases of AE is small, but still underreported, and is likely to increase in the near future [Reference Romig4].
In The Netherlands, E. multilocularis was initially detected in three foxes in 1996 near the Belgian border, and the parasite was shown to have been spreading among local fox populations in a northerly direction in Limburg following its likely introduction at the border with Belgium [Reference Takumi5]. The likely event of the introduction and subsequent spread of the parasite in the local fox population raised concern regarding the increased potential of human risk of AE in the coming years.
In order to obtain insight into the risk for human AE in The Netherlands, we used an approach where we assumed a relationship between eggs shed into the environment by definitive hosts and the risk of disease in humans. Since almost no data exist that help to quantify this relationship, we hypothesized that the number of AE cases in Switzerland has increased in recent years due to an increasing number of parasite eggs in the environment, following primarily an increasing fox population density. Given this hypothesis, the risk of AE for humans and the incubation period were estimated based on human-, fox- and parasite-related datasets compiled in Switzerland. Based on the estimated risk, we constructed a risk map for Limburg using the fox and parasite burden datasets from Limburg, and local human population densities. Assuming that the parasite continued to spread in fox populations in Limburg at a speed that was estimated from the surveillance results for 1996 and 2003 [Reference Takumi5], we were able to predict time-courses of AE epidemiology in Limburg up to 2018.
Historical time-series of AE in Switzerland and in the Canton of Zurich were recorded in the database. Swiss human populations were recorded between 1950 and 2005. The numbers of foxes shot nationwide in the period between 1950 and 2007 were recorded into hunting statistics. For parasitological examination, foxes (n=799) were collected between 1996 and 2001 in the city of Zurich (urban and peri-urban area). The numbers of E. multilocularis in foxes were determined by the intestinal sedimentation and counting technique according to Hofer et al. [Reference Hofer6] and recorded in the database.
The number of parasite eggs in the environment will increase by the shedding of eggs originating from infected foxes and will decrease by inactivation, e.g. by environmental conditions. Because most eggs are inactivated within one year [Reference Veit7], the mean number of parasite eggs (g) in a unit area is assumed to be proportional to the mean number of patent parasites in the intestines of the fox population in that area:
The constant of proportionality is equal to the mean number of parasite eggs produced during the adult worm's lifespan (a). The symbol m is the mean number of adult parasites per fox. N is the mean fox population density/km2.
The number of adult parasites per individual fox is empirically described by the negative binomial distribution [Reference Takumi5]. The probability that a fox is infected with a number of adult worms (i) is,
when the mean fox population density is equal to N, the distribution is still negatively binomial but with the mean multiplied by N. The parameter k indicates an aggregation in the number of adult parasite worms in the intestines of the fox population. Smaller values for k indicate that a small fraction of foxes accounts for the majority of the total adult parasite biomass.
To relate the risk of human AE to the number of parasite eggs in the environment, we assumed that a single parasite egg in the environment may be ingested by a person, the egg is able to infect a person after ingestion and the infection is able to subsequently cause AE. The probability that an individual acquires AE is denoted by γ. Processes represented by the probability γ are thus ingestion of a single parasite egg from the environment by a person, infection of the person after ingestion, and the development of AE in the same person. When the number of parasite eggs in the environment is negatively binomially distributed, the probability of acquiring human AE is
Thus, the probability of infection increases by an increase in the number of ingested eggs.
Because of a lengthy incubation period, AE may develop many years after ingestion. The incubation period is variable and it is assumed to be Poisson-distributed with the mean τ. The probability that the incubation period is equal to j is,
The mean number of adult parasites per fox (m) and the aggregation parameter (k) were estimated based on the adult parasite counts of the foxes (worm burden) collected in and near the city of Zurich. Estimates were obtained by maximizing the likelihood:
The multiplication of the negative binomial distribution f [equation (1)], is performed over all foxes examined (n=799). In addition to estimating the parameters of the negative binomial distribution for the Canton of Zurich, we assessed whether the mean worm burden per fox changed over the period 1996–2001 by employing χ2 tests. P values <0·05 were considered to represent significant change in the mean worm burden.
The average number of parasite eggs produced during the lifespan of an adult parasite (a) was estimated to be 27 eggs based on experimental infection of foxes [Reference Kapel8]. The egg production per adult parasite (a) is assumed to be constant over the period during which Swiss AE datasets were collected.
Since it is difficult to estimate the fox population density, we used the numbers of shot foxes in Switzerland as proxy for the population density [source: Swiss hunting statistics, 2009 (http://www.wild.uzh.ch/jagdst/index.php)]. We therefore divided the historical records of numbers of foxes shot in Switzerland in the period 1950–2007 [Reference Schweiger2] by the area of Switzerland (39 770 km2). The resulting proxy values for the mean fox population density/km2 in Switzerland ranged from 0·3/km2 in the 1980s to 1·1/km2 in 2000s.
Since the incubation period of AE has a wide range, AE may develop many years after ingestion of the parasite's eggs. Therefore, a long and variable incubation period was taken into account in calculating the probability of AE in the year t by the following equation.
The probability of disease P AE is now explicitly written to depend on the year t, as it changes with the annual fox density data. The incubation period is assumed to be between 1 and 20 years. The probability of more than 20 years is considered zero.
The numbers of AE cases reported in the Canton of Zurich each year was modelled as a binomial process. Estimates for the probability of disease (γ) caused by a parasite's egg and the mean incubation period (τ) were obtained by maximizing the likelihood,
The multiplication is performed over the years 1966 to 2005. The symbols represent the probability of acquiring AE per person Q t [equation (2)], the numbers of AE cases reported in the Canton of Zurich per year V t, and the population size of the Canton of Zurich Z t. The human population of the Canton of Zurich was estimated to be 17·5% of the total Swiss population. In order to obtain missing population sizes for some years prior to 1995, we interpolated the Swiss population data by spline.
The province of Limburg in The Netherlands was partitioned into 1 km2 square grids. At each grid, the probability (i.e. risk) of acquiring AE per person per year was calculated by substituting the Limburg-specific estimates for fox density and parasite burden into equation (2). The probability of infection (γ) estimated from the Swiss datasets were substituted into equation (2). With this approach, we made three assumptions. First, Swiss and Dutch individuals are equally susceptible to infection upon ingesting one parasite egg. Second, infected Swiss and Dutch individuals are equally likely to develop AE. Third, Swiss and Dutch AE patients are equally likely to be diagnosed. The first and second assumptions are justified because of the same European human origin. Regarding the third assumption, a small-scale serosurveillance in 2010 in Limburg did not detect any evidence of exposure to parasite eggs in the local human population. Thus under-diagnosis by local GPs can be excluded at this time point. The mean incubation period (τ) estimated from the Swiss datasets was substituted into equation (2) to take into account the incubation period in the epidemiology of AE in Limburg.
The mean worm burden per fox in Limburg was calculated using a mathematical model [Reference Takumi5] describing the dynamics and the spread of E. multilocularis in the population of foxes in Limburg. The mean parasite burden per fox were calculated for each year between 1996 and 2018 and at each grid in Limburg, and subsequently stored in a database for the calculation of the risk of human AE in Limburg. If the mean worm burden exceeded 1000 adult parasites per fox, it was set to 1000. Here we assumed that the mean worm burden in foxes in the endemic region of Switzerland was the upper limit of the mean. The parasite burden per fox was assumed to be zero before 1996 in Limburg, because E. multilocularis has never been found in The Netherlands in foxes prior to 1996 [Reference Borgsteede9].
As proxies for the local fox densities, we obtained the numbers of foxes shot in the province of Limburg in the period 2000–2001 for each 1 km2 square (M. Montizaan, Koninklijke Nederlandse Jagers Vereniging, personal communication). The densities of shot foxes ranged between 0·11/km2 and 3·57/km2 (mean±s.d.=1·46±0·94/km2). At some locations, the number of hunted foxes was not reported. The density at those locations was set to 1 fox/km2 in order to avoid superficial zero-risk. The local fox population densities were assumed to be constant for the period 1996–2018, although this might give an underestimation of the risk, since fox population densities are increasing in some parts of Europe [Reference Schweiger2].
Human population density/km2 in Limburg was computed from the numbers of residents per six alphanumeric postcodes (Planbureau voor de leefomgeving) using ArcGIS 9.3. To model the exposure of humans to the parasite's eggs at locations that are potentially different from the residential address, we applied a Gaussian filter (Mathematica, Wolfram Research Inc., USA) with radius 30 km to the address-based human population density/km2. The human population densities were assumed to be constant over the years.
For the period 2008–2018, a sample was drawn from the Poisson distribution with the mean number of AE cases from each grid in Limburg for each specific year. The mean number of AE cases for a specific year was calculated by multiplying the risk of human AE for that year [equation (2)] with the exposure-based human population density on a per grid basis. One simulation run represents the epidemiology of AE in Limburg for the period 2008–2018. The simulation was repeated 1000 times to construct the final risk map.
According to our analyses, foxes in the Canton of Zurich were infected with 1070 adult parasites on average. As reported previously [Reference Takumi5, Reference Hofer6], the majority of adult parasites were present in a small number of foxes. In the 5-year period between 1996 and 2001, the mean worm burden per fox in the Canton of Zurich remained constant (P<0·62). The risk of an individual acquiring AE in the Canton of Zurich is estimated to be 2·65×10−9 per egg present in the environment. The estimated incubation period is 8·1 years in the Canton of Zurich. When all the AE cases reported nationwide in Switzerland were analysed, the incubation period was 11 years (3 years longer than the Canton of Zurich) but the risk of acquiring AE was the same. The registered numbers of human AE cases in the Canton of Zurich were enclosed between the predicted 95% confidence interval (CI) of annual human AE cases in this canton for each year in the 40-year period between 1966 and 2005. In only one instance, in 2001, did the registered cases exceed the 95% CI, thereby validating the modelling approach for the purpose of risk assessment in Limburg. This successful validation indicates that our postulate alone (i.e. a direct relationship exists between the parasite's eggs and the disease risk) is sufficient to explain the local and national differences in the reported cases of AE, and differences in human behaviours that might make some individuals more prone to acquire AE than others [Reference Kern10], are not necessary to assume.
Based on the estimated risk of AE in Switzerland and the incubation period, the total number of AE cases in Limburg was predicted up to the year 2018. By 2018, 0·9 case of AE is expected (95% CI 0–3 cases). Most AE cases were sampled during simulations at one case/km2 but at some locations cases were sampled multiple times. The risk of acquiring AE appears to be higher in the east and the south of the city of Maastricht (Fig. 1).
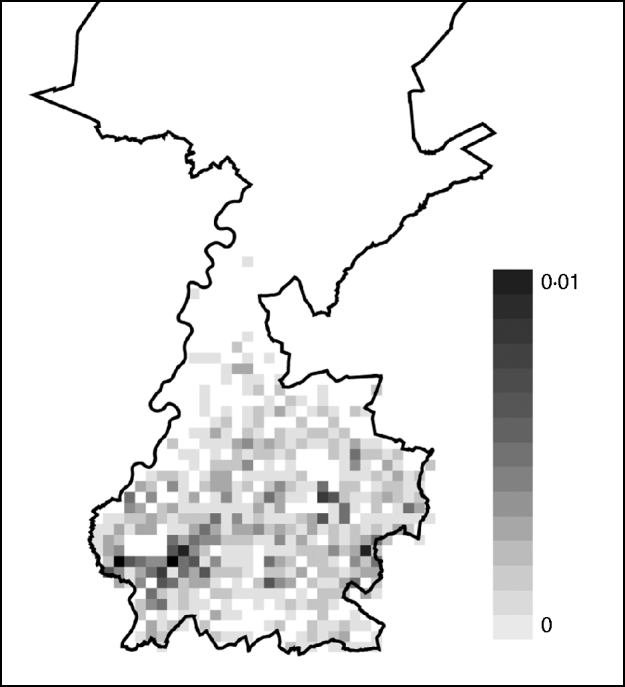
Fig. 1. Risk map of alveolar echinococcosis (AE) in Limburg up to the year 2018. Cases of AE sampled in a total of 1000 simulation runs were added at each square and averaged by dividing the total by 1000. The resulting average cumulative numbers ranged between 0 and 0·01 cases per square and the quantities are illustrated in the scale shading from white to black. A square is 1 km2. The cumulative number of AE over the depicted region of Limburg was 0·9 cases.
In this study, we developed a method to quantify the relationship between the amount of eggs in the environment and the probability of infection in humans and used it to predict the future epidemiology of AE in The Netherlands. In The Netherlands, E. multilocularis is now an established parasite within the local fox population, and consequently, there is a need to assess the human risk of this potentially fatal disease. Until recently, it was not possible to quantify the human risk because no relationship was known between the estimated number of parasite eggs in the environment and the probability of infection in humans. In this study, we were able to use the datasets from a recent Swiss study to relatively quantify this relationship.
In 2008, the first AE patient in The Netherlands was reported [Reference van Dommelen11]. The patient is a resident of Kerkrade in the province of Limburg. Risk factors of AE such as hunting, growing vegetables and gardening were not present for this patient. The patient spent three short vacations in Switzerland, Italy, and Austria in 2006 and in 2007, where exposure to parasite eggs could have occurred. However, the possibility that the patient ingested parasite eggs locally in Limburg could not be excluded since Limburg is one of the areas where the parasite is spreading in the fox population and the environment. We speculate that the first case of AE might have been foreseen by the events that took place in the past in the wildlife population.
In conclusion, our modelling approach indicates that the epidemiology of AE in The Netherlands might have changed from a period of negligible risk in the past to a period of increasing human risk in the coming years. We estimated that the number of AE cases will increase up to three cases by 2018 based on the assumptions made, and a further increase in the number of autochthonous cases in Limburg beyond 2018 is very likely. The future estimated numbers of expected cases in Limburg will have consequences for the policy concerning zoonotic disease control in this region. Moreover, our approach could be applicable to other regions in Europe where E. multilocularis is present.
DECLARATION OF INTEREST
None.



