INTRODUCTION
Despite the development of effective antibiotics and supportive care, sepsis remains an unconquered challenge for clinicians with an unacceptably high morbidity and mortality rate in critically ill patients [Reference Martin1]. Early intervention to prevent subsequent or worsening clinical deterioration is a key to the successful treatment of patients with potential sepsis. Two major factors which influence the effectiveness of sepsis treatment strategies are the ability to recognize the early stages of the disease and to estimate its severity. It is often difficult to determine which patients with signs of infection on initial evaluation have, or will develop, more severe illness. Specifically, the development of organ dysfunction portends a poor outcome and is a common pathway to death in these patients. Therefore, predictive markers to identify high-risk patients are urgently needed for early detection and preventive care of sepsis. Recently, there has been increasing evidence supporting the view that genetic factors, particularly single nucleotide polymorphisms (SNPs), influence individual susceptibility and severity of sepsis [Reference Namath and Patterson2]. Delineating the variation in genes and associated differences in response to sepsis might contribute to the development of new genetically tailored diagnostic and therapeutic interventions that will improve outcome in the patients with sepsis.
The mannose-binding lectin (MBL), an important component of the complement system, is a calcium-dependent collagenous lectin that is secreted by the liver as an acute-phase protein. It plays an important role in innate immunity by recognizing and binding to sugar moieties on the surface of bacteria, viruses, fungi, and parasites. MBL binding causes these microorganisms to agglutinate and allows phagocytic clearance of pathogens as well as activation of the lectin-complement pathway, through MBL-associated serine proteases (MASP)-1 and -2 [Reference Turner3]. Low concentrations of MBL cause defects in opsonization and phagocytosis that have been associated with recurrent and serious infections in both infants and adults [Reference Koch4, Reference Summerfield5]. Furthermore, experiments with ‘knockout’ MBL null mice have indicated that the MBL-complement pathway has a non-redundant role in defence against bloodborne Staphylococcus aureus infection [Reference Shi6].
The human MBL gene is located on chromosome 10 (10q11·2–q21). About 30 genetic variants have been identified so far within the entire MBL gene from the HapMap database. However, only five have been well studied in relation to their clinical relevance, they are –550 (rs11003125G/C, termed H/L), –221 (rs7096206G/C, termed Y/X) in the 5′-flanking region and codons 52 (rs5030737C/T, termed A/D), 54 (rs1800450G/A, termed A/B), 57 (rs1800451G/A, termed A/C) of exon 1 (the three variant alleles collectively termed O and the wild-type allele termed A). MBL production is controlled by the MBL gene and its polymorphisms. The presence of key structural and promoter variants is reasonably well correlated with reduced serum MBL levels, and genotypic analyses are used frequently as surrogates for MBL serum levels [Reference Gordon7, Reference Eisen8]. Individuals heterozygous for these polymorphisms (A/O) have reduced circulating MBL levels, whereas in those who are homozygous or compound heterozygous (O/O), circulating MBL is almost absent. Further, the presence of MBL variant alleles and low levels of serum MBL have been closely associated with the development of sepsis and fatal outcome in adult patients admitted to intensive care [Reference Gordon7, Reference Huh9] and in children [Reference Ozkan10]. Nevertheless, studies have shown inconclusive or contradictory results [Reference Israels11] as most studies had relatively small sample sizes and thus lacked the statistical power to resolve association of racial or ethnic factors, or uncorrected multiple hypothesis testing.
Meta-analysis is a means of augmenting the effective sample size by pooling data from individual association studies, thus enhancing the statistical power of the analysis for the estimation of genetic effects. Therefore, the purpose of this study was to investigate whether MBL polymorphisms were associated with an increased risk of sepsis or higher sepsis mortality. To our knowledge, this is the first meta-analysis of the association between MBL polymorphisms and susceptibility or progression of sepsis.
MATERIALS AND METHODS
Identification and eligibility of relevant study
Relevant articles were identified through a literature search using the keywords ‘MBL or mannose binding lectin or mannose binding protein’ and ‘sepsis or severe sepsis or septic shock’ and ‘polymorphism or genetic variant or mutation’ in Pubmed, EMBASE, and Web of Knowledge databases (published up to 7 May 2013). All the searched articles were retrieved, and their references were checked for other relevant publications. Review articles were also searched to find additional eligible studies. The following criteria were used for inclusion of identified articles for our meta-analysis: (a) evaluation of all MBL gene polymorphisms and sepsis, (b) case-control study or cohort design, (c) studies that contained available genotype frequencies and (d) studies written in English. The major reason for exclusion of articles was if they were reviews, comments, or the absence of usable data by contacting the studies’ authors. For overlapping studies, only the one with the largest sample size was included.
Data extraction and methodological approach
To minimize bias and improve reliability, two researchers (A.-Q.Z. and C.-L.Y.) extracted data with the above inclusion and exclusion criteria independently and reached a consensus. Information such as the first author's name, publication year, country and ethnicity of study population, genotyping method, sepsis type, age, genotype number or allele frequency for cases and controls was collected from each study using a standardized data collection protocol. For studies including subjects of different populations, data were extracted separately.
Qualitative assessment
Two researchers (W.P. and J.-W.G.) independently assessed the quality of each study. Any disagreement was resolved by consensus. Quality assessment scores of genetic association studies of human sepsis were used to assess the quality of the selected articles [Reference Clark and Baudouin12]. This quality scoring system was based on both traditional epidemiological considerations and genetic issues. Total scores ranged from 0 (worst) to 9 (best) for cohort studies and 0 (worst) to 10 (best) for case-control studies.
Meta-analysis
The strength of the association between MBL polymorphisms and sepsis was measured by odd ratios (ORs) with 95% confidence intervals (95% CIs) for dominant (BB+AB vs. AA), recessive (BB vs. AB+AA), and allelic (B vs. A) genetic models, respectively (A represented major allele, B represented minor allele). Because we anticipated heterogeneity between studies, a random-effects model, using the Mantel–Haenszel method, was used to calculate the pooled ORs for all analyses. The statistical significance of ORs was determined using the Z test. Consistent with common practice, we also performed a statistical test for heterogeneity using Cochran's Q test and quantified the degree of heterogeneity with the I 2 statistic [Reference Higgins13].
Departure from Hardy–Weinberg equilibrium (HWE) in controls was tested by the χ 2 test. Subgroup analyses were conducted according to ethnicity (Caucasian, Asian), type of sepsis (sepsis, severe sepsis, septic shock) and age group (adult, paediatric). To assess the stability of the results, sensitivity analysis was performed through sequentially excluded individual studies. Moreover, sensitivity analysis was also performed, excluding studies whose allele frequencies in controls exhibited significant deviation from HWE, given that the deviation may denote bias [Reference Thakkinstian14]. The potential publication bias was examined visually by Begg's funnel plot of log OR against its standard error (s.e.), and the degree of asymmetry was tested using Egger's test.
All statistical tests were performed using Review Manager 5.2 (The Cochrane Collaboration, UK) and Stata v. 11.0 software (StataCorp LP, USA). A P value <0·05 was considered statistically significant, except for the tests of heterogeneity where a level of 0·10 was used.
RESULTS
Characteristics of eligible studies
A total of 230 articles was found up to 7 May 2013 (55 from Pubmed, 58 from EMBASE, 117 from Web of Knowledge), but only 38 articles were preliminarily identified for further evaluation. After carefully evaluating the quality of the remaining articles 13 were excluded, three of which [Reference Abu-Maziad15–Reference Sutherland, Walley and Russell17] did not supply detailed genotype data after contacting the studies’ authors, six articles were conference abstracts and four articles were comments. Moreover, an additional five articles were included through citations in other articles. Finally, 30 relevant articles addressing four polymorphisms in MBL gene were chosen for the study (Fig. 1).
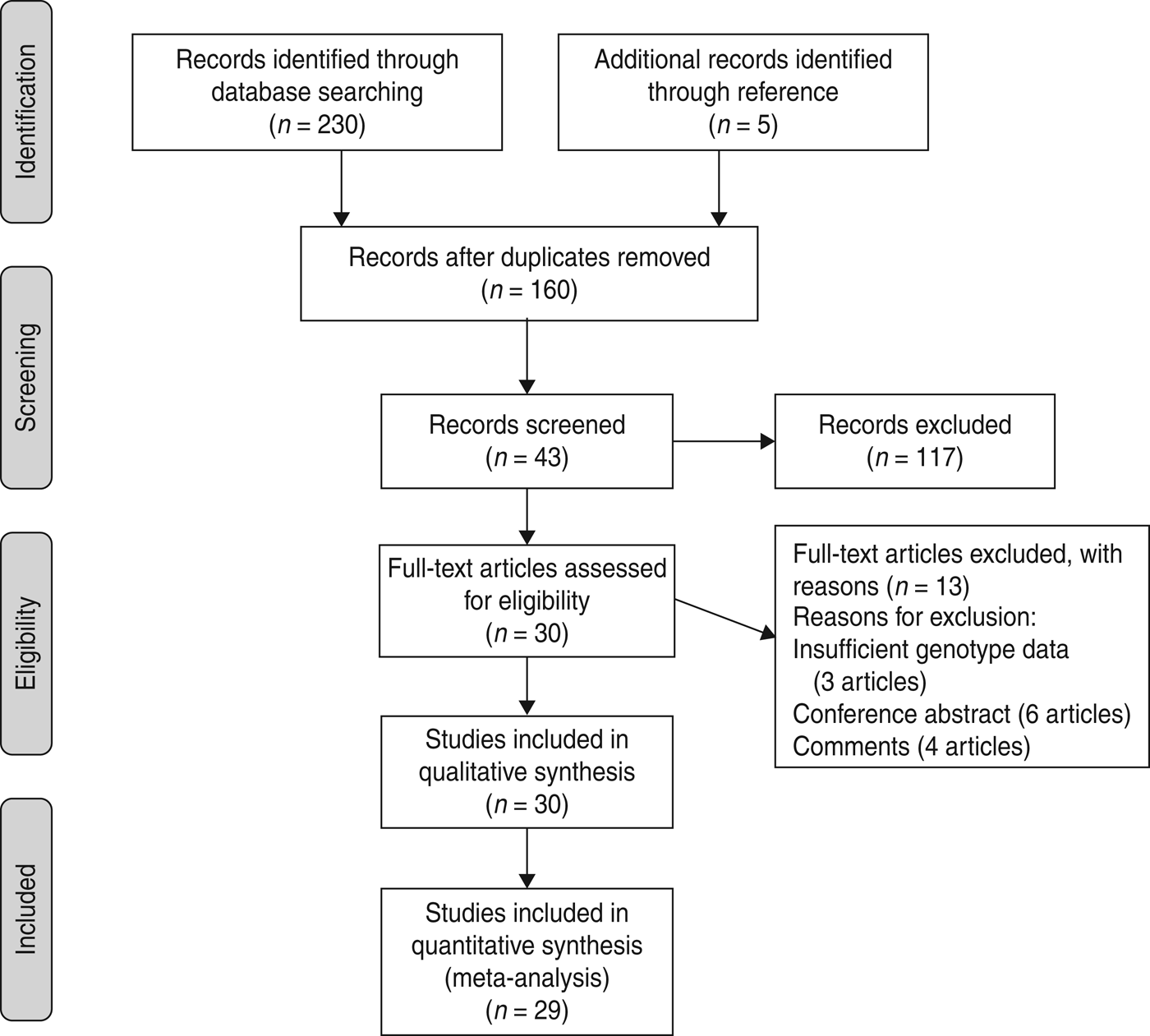
Fig. 1. Flow of study identification, inclusion, and exclusion.
Information of the 30 chosen articles included the first author, publication year, country and ethnicity, sepsis type, age, sample size (case/control), genotype data, and quality score (Table 1). The majority of the included studies were performed in Caucasian populations and only two studies were in Asian populations [Reference Huh9, Reference Horiuchi18]. Twenty studies were focused on adults and ten on paediatric populations; 29 studies evaluated susceptibility to sepsis and one addressed sepsis mortality [Reference Garnacho-Montero19]. The quality scores of most studies ranged from 5 to 8, suggesting high quality.
Table 1. Characteristics of the included studies
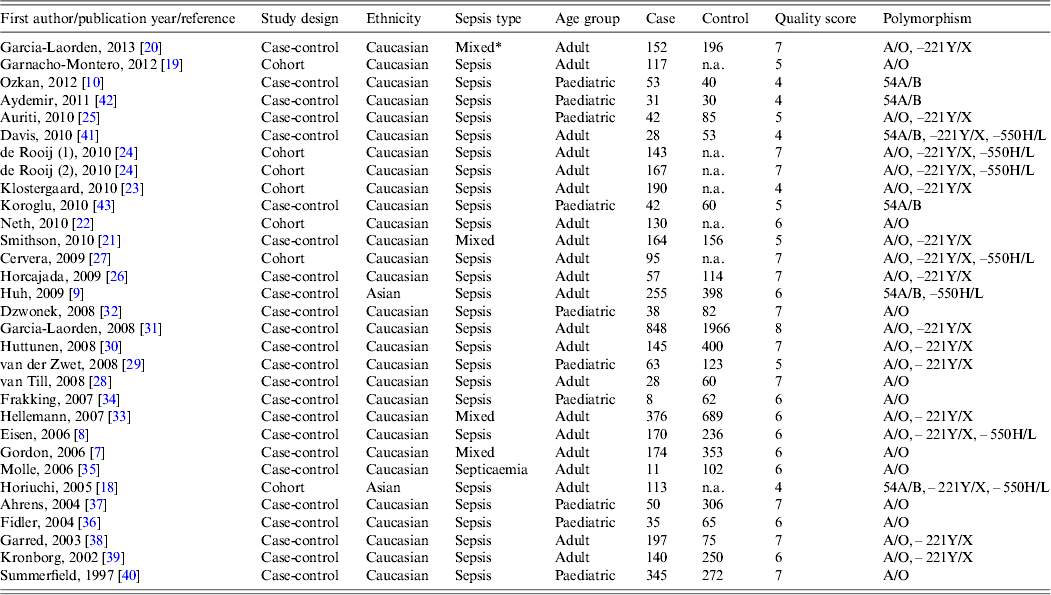
n.a., Not available.
* Mixed includes the conditions sepsis, severe sepsis or septic shock.
Various different genotyping methods were used in the studies, including the classical polymerase chain reaction–restriction fragment length polymorphism (PCR–RFLP) in nine, TaqMan probes in seven, direct sequencing in six, heteroduplex analyses in four, sequence-specific primer PCR (SSP-PCR) in three and high-resolution DNA melting assay (HRMA) in one study.
Quantitative data synthesis
Structure variant A/O polymorphism
Twenty-four studies [Reference Gordon7, Reference Eisen8, Reference Garcia-Laorden20–Reference Summerfield40] containing 3371 sepsis patients and 5989 controls were identified as investigating MBL A/O polymorphisms located in the coding region and their association with susceptibility to sepsis, which were all performed in Caucasian populations. There were significant associations in overall comparison and subgroup analysis by the dominant and allelic genetic models. The structure variant O allele increased the overall risk of sepsis (dominant model: OR 1·27, 95% CI 1·05–1·52, P = 0·01; I 2 = 68%, P < 0·01; allelic model: OR 1·19, 95% CI 1·02–1·40, P = 0·03; I 2 = 68%, P < 0·01) (Figs 2, 3). Based on different age groups, association was also found in paediatric populations (dominant model: OR 1·72, 95% CI 1·16–2·56, P = 0·007; I 2 = 55%, P = 0·04). However, no significant association was found in adults (Table 2). In addition, only one study by Garnacho-Montero et al. [Reference Garnacho-Montero19] reported that MBL AO/OO was associated with in-hospital mortality in 117 pneumococcal sepsis patients [adjusted hazard ratios (aHR) 3·2, 95% CI 1·01–9·8, P = 0·048] after controlling for confounding variables.

Fig. 2. Meta-analysis for the association between susceptibility to sepsis and the MBL A/O polymorphism (dominant model: OO + AO vs. AA).

Fig. 3. Meta-analysis for the association between susceptibility to sepsis and the MBL A/O polymorphism (allelic model: O vs. A).
Table 2. Summary of meta-analysis results
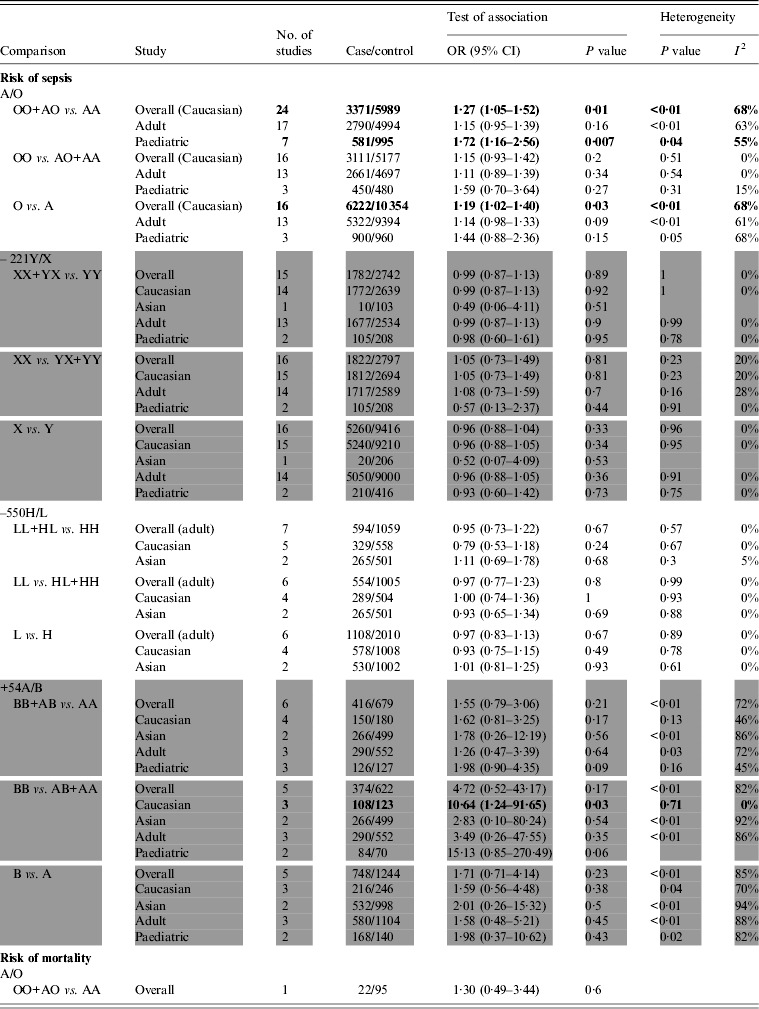
OR, Odds ratio; CI, confidence interval.
–221Y/X, –550H/L and +54A/B polymorphisms
Seventeen studies [Reference Eisen8, Reference Horiuchi18, Reference Garcia-Laorden20, Reference Smithson21, Reference Klostergaard23–Reference Cervera27, Reference van der Zwet29–Reference Garcia-Laorden31, Reference Hellemann33, Reference Garred38, Reference Kronborg39, Reference Davis41] evaluated –221Y/X and its association with susceptibility to sepsis (2670 cases and 4763 controls). Seven studies [Reference Eisen8, Reference Huh9, Reference Horiuchi18, Reference de Rooij24, Reference Cervera27, Reference Davis41] and six [Reference Huh9, Reference Ozkan10, Reference Horiuchi18, Reference Davis41–Reference Koroglu43] evaluated the association between –550H/L, +54A/B and susceptibility to sepsis, respectively. Our analysis did not provide any statistical evidence of association between –221Y/X, and –550H/L polymorphisms and susceptibility to sepsis (Table 2). However, for +54A/B, which was one of the three codon variations in exon 1 of the MBL gene, there was a slight association between BB genotype and susceptibility to sepsis in the recessive genetic model (OR 10·64, 95% CI 1·24–91·65, P = 0·03; I 2 = 0%, P = 0·71) in Caucasian populations. However, no association was found in Asian populations and for all populations overall.
Heterogeneity analysis
For the A/O polymorphism, significant between-study heterogeneity was found in overall comparisons in the dominant and allelic genetic models (I 2 = 68%, P < 0·01). The heterogeneity was not obviously decreased by subgroup analysis and sequentially excluding each study. The results of meta-regression for the A/O polymorphism indicated that publication year and age group of study population independently contributed to the observed heterogeneity. Effects of publication year on heterogeneity were significant by the dominant and allelic models (dominant: P = 0·057<0·1; allelic: P = 0·078<0·1). Genotyping methods and sample size were not statistically associated with heterogeneity.
For the +54A/B polymorphism, heterogeneity between studies was statistically significant by any genetic model (Table 2) in overall comparison. I 2 decreased and P value exceeded 0·10 for Caucasian or paediatric subgroups, respectively. For –221Y/X and –550H/L polymorphisms, there was no obvious heterogeneity in any genetic models by both overall comparison and subgroup analysis.
Cumulative meta-analysis
Cumulative meta-analyses of the two significant associations of the A/O polymorphism were performed via the assortment of studies by publication time. As shown in Figures 4 and 5, inclinations toward significant associations were evident with each addition of more data over time. The results showed that the pooled ORs tended to be stable.

Fig. 4. Cumulative meta-analysis of association between the MBL A/O polymorphism and susceptibility to sepsis (dominant model: OO + AO vs. AA).
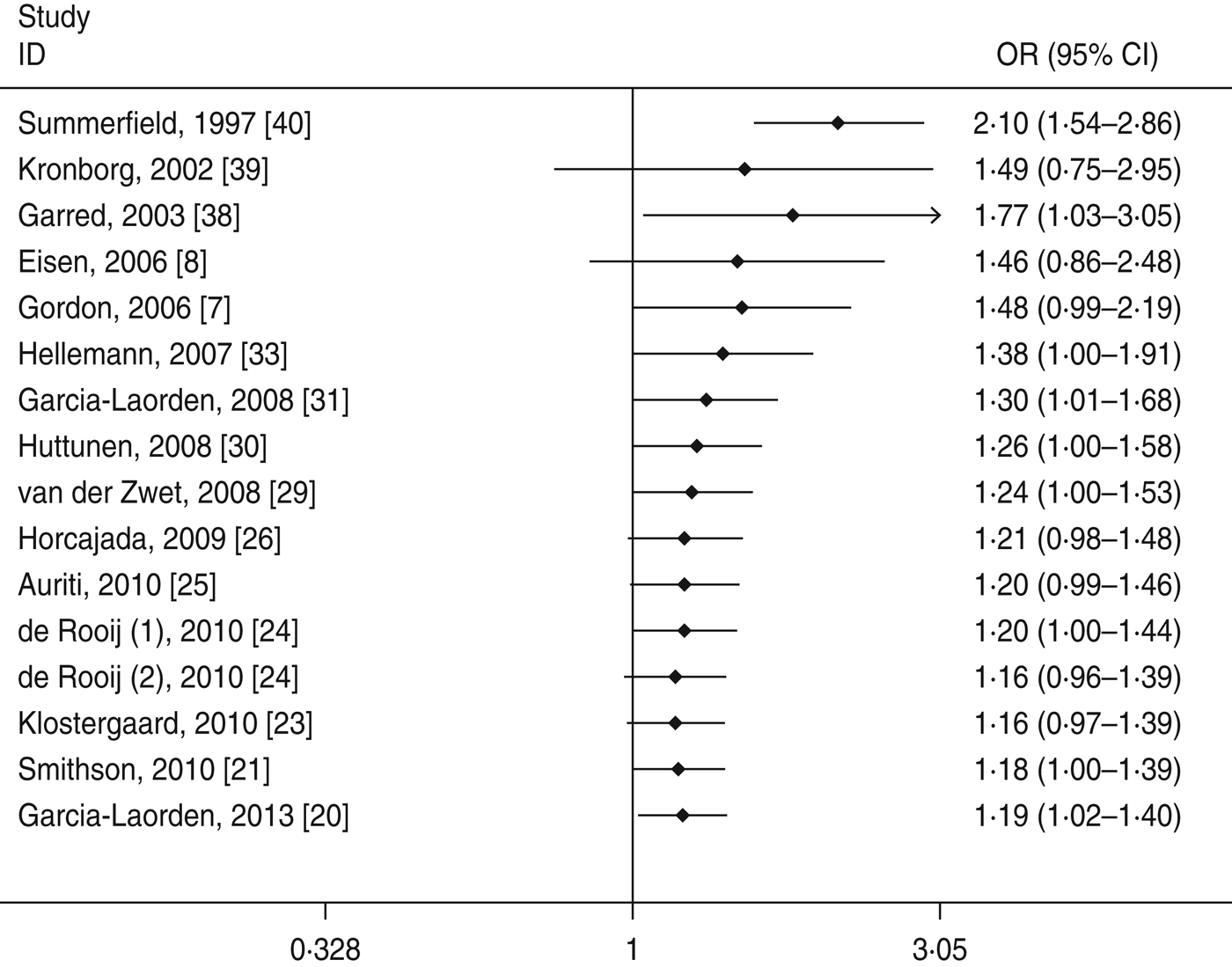
Fig. 5. Cumulative meta-analysis of association between the MBL A/O polymorphism and susceptibility to sepsis (allelic model: O vs. A).
Publication bias
Publication bias was examined by Begg's funnel plots qualitatively and estimated by Egger's test quantitatively for all genetic models. If the number of included studies was small, it was unnecessary to perform publication bias analysis. After combining all studies, slight asymmetry was observed for the A/O polymorphism in the dominant and allelic genetic models (Fig. 6), but Egger's test did not show evidence of publication bias (dominant: P = 0·283; allelic: P = 0·433). For –221Y/X, funnel plots were symmetrical and Egger's test for all models showed no significance, suggesting little evidence of publication bias.
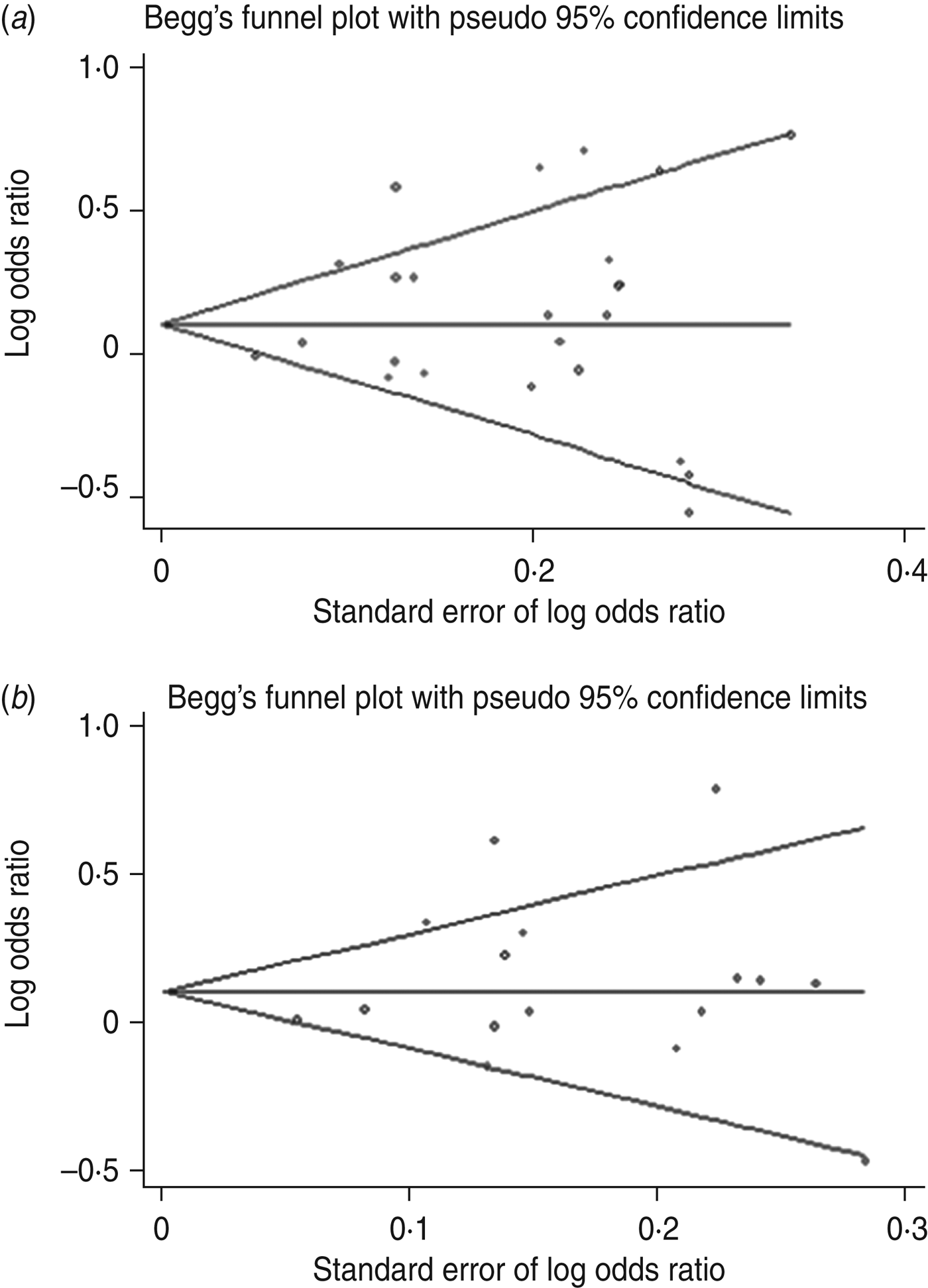
Fig. 6. Begg's funnel plot with pseudo-95% confidence limits for susceptibility to sepsis and the MBL A/O polymorphism. (a) dominant model (OO + AO vs. AA), (b) allelic model (O vs. A).
DISCUSSION
In this comprehensive meta-analysis, MBL structure variant alleles were shown to increase the susceptibility to sepsis, whereas –221Y/X, and –550H/L showed no association with the development of sepsis. Previous studies have reported that the MBL was implicated in sepsis susceptibility and severity through altered circulating levels and activity, which were influenced by the MBL polymorphisms. The three structure variants at codons 54, 57, and 52 result in amino-acid substitutions that interfere with assembly of functional oligomers, thereby decreasing circulating levels, compromising ligand binding, and reducing complement activation. Individuals heterozygous for these polymorphisms (A/O) have reduced circulating MBL levels, whereas for those who are homozygous or compound heterozygous (O/O), circulating MBL is almost absent [Reference Gordon7]. In our meta-analysis, the A/O polymorphism was a risk factor for developing sepsis in the overall study population. Carriers of the O allele (OO+AO) had a 27% increased susceptibility to sepsis compared to individuals carrying an AA genotype. Because all studies of the A/O polymorphism were in Caucasian populations, more studies in Asian populations are needed to evaluate the effect of this polymorphism on susceptibility to sepsis.
In the subgroup analysis, we observed that paediatric patients who carried an O allele had an increased susceptibility to sepsis (dominant: OR 1·72, 95% CI 1·16–2·56, P = 0·007), which should be confirmed in a larger sample population. However, the result was not statistically significant in the adult group (OR 1·15, 95% CI 0·95–1·39, P = 0·16). Results from different age groups were inconsistent, which might be interpreted as indicative of a genetic predisposition to sepsis and is further modulated by both genetic (gender) and non-genetic (age) factors [Reference Watanabe44]. In addition, only one study [Reference Garnacho-Montero19] revealed that, after controlling for confounding variables, structural variant alleles O in the MBL gene were independently associated with mortality. Because a relatively small sample size may have insufficient statistical power to detect a slight effect, these associations require further studies to confirm.
The –550G/C and –221G/C polymorphisms of the MBL gene promoter region are also known to affect the transcription level of MBL, and the X allele represents the MBL level similar to those with structural variants at codons 52, 54, and 57. There was no significant difference in the incidence of sepsis with H/L or X/Y alleles in the overall comparison and subgroup analysis. However, as there are only seven studies for –550H/L, such negative results do not rule out an association with susceptibility to sepsis.
The inconsistency of results for different MBL polymorphisms for susceptibility to sepsis can be explained by several reasons. First, the study population in each report originates from different geographical areas and races. Different genetic backgrounds and environmental factors could influence the frequency of polymorphisms. Second, the small sample size in some studies contributes to inconsistency and it is necessary to study larger sample sizes to decrease the possibility of false-positive and false-negative associations. Third, inconsistency in the composition of control populations in studies may also influence the observed associations with susceptibility to sepsis. Finally, some cases are paediatric patients and the development of sepsis could be modulated both by genetic polymorphisms and age difference factors.
Heterogeneity is an important factor in the interpretation of the results of a meta-analysis. In this study, significant heterogeneity was found in the structure variations. For the A/O polymorphism, meta-regression demonstrated that age group and publication year might be the major source of the heterogeneity. Moreover, in a sensitivity analysis to evaluate the stability of the meta-analysis the removal of each study did not appear to alter the observed associations with susceptibility to sepsis and heterogeneity, suggesting the reliability of these results. The cumulative meta-analyses showed a trend of more marked associations between the MBL A/O polymorphism and increased susceptibility to sepsis as data accumulated each year. This procedure also proved that our results were robust.
Salanti et al. [Reference Salanti, Sanderson and Higgins45] suggested that false-negative results may be suppressed or false-positive results magnified and thus the results of meta-analyses might be influenced by publication bias. Although Egger's test did not show significant publication bias for susceptibility to sepsis, the shape of the funnel plot was slightly asymmetrical and so the results should be interpreted cautiously and further studies are warranted to confirm these findings.
There are some limitations in our analysis. First, although we collected all eligible studies, the sample size of the included studies was often insufficient, which could increase the likelihood of type I and type II errors. Therefore, there was a lack of statistical power to better evaluate the association between MBL polymorphisms and susceptibility to sepsis, especially in subgroup analysis. Second, we showed the results by combining all studied populations; however, the results of subgroup analysis were more meaningful. We only analysed the data based on different age groups and ethnicity of the studied populations due to the limited data. Third, our analysis was primarily based on unadjusted effect estimated and therefore the potential covariates including comorbidities, sepsis severity, duration of hospitalization, surgery, pathogens, sex, and source of controls were closely related to susceptibility or progression of sepsis and could not be controlled due to limited information in the original studies. Fourth, although Egger's test did not show any obvious publication bias, the influence of bias in the present analysis could not be completely excluded as studies with positive results are more easily published than those with negative results, and only studies published in English were included. Finally, as we only focused on the associations of MBL polymorphisms, the significance was limited and to ensure the validity and reliability of the conclusions, it would be necessary to perform a meta-analysis on the associations between sepsis-related mortality vs. sepsis controls in a future study. There is also a need to examine the effect of other and multiple polymorphisms on susceptibility to sepsis.
In conclusion this meta-analysis demonstrates that the A/O polymorphism of MBL has a significant association with susceptibility to sepsis, and no such association exists between –550H/L, and –221Y/X. Further homogeneous studies with larger sample sizes are needed to evaluate other associations in order to identify suitable targets for diagnosis and intervention of sepsis.
ACKNOWLEDGEMENTS
This work was supported by National Key Technology R&D Programme (2012BAI11B01), the Major State Basic Research Development Programme of China (2012CB518104), and National Natural Science Funds for Distinguished Young Scholar (81201462). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
DECLARATION OF INTEREST
None.











