Introduction
Throughout military history, acute gastrointestinal illness (AGI) has been a significant cause of morbidity and mortality among US service members [1]. Diarrhoeal disease caused more deaths than enemy action during the Revolutionary War and during the Civil War diarrhoeal disease occurred with more frequency and produced more sickness and mortality than any other form of the disease [1, Reference Keaton2]. Symptoms of AGI include diarrhoea, vomiting, fever, malaise and/or weakness. Not only can AGI affect individual medical readiness, if a large proportion of the military population is affected by AGI, military operational effectiveness can be degraded [Reference Sanders3].
Despite advances in medicine and improvements in basic sanitation, modern day military operations continue to be affected by gastrointestinal illness. During Operation Desert Shield, 57% of surveyed troops reported at least one episode of diarrhoea, while 20% reported they were temporarily unable to perform duties due to their symptoms [Reference Hyams4]. In 2012, diarrhoeal diseases caused more than 17 000 healthcare encounters affecting over 15 000 US service members [5]. In 2018, an outbreak of AGI caused by Shiga toxin-producing Escherichia coli, a major cause of foodborne illness, sickened 244 male recruits at the Marine Corps Recruit Depot, 15 of which had life-threatening complications[Reference Keaton2]. Historically and recently, AGI is a critical impediment to military readiness.
To our knowledge, a rigorous attempt to estimate the true incidence of AGI among the nondeployed active duty US Army military population has never been attempted. This is a considerable gap in knowledge as AGI burden measures are necessary for directing policy and interventions aimed at reducing preventable causes of AGI (e.g. foodborne disease). Estimating the number of AGI among US Army service members is challenging for three reasons: deficient military-specific active surveillance systems, underreporting and underdiagnosis. We will speak to these in order.
The US Army has no military gastrointestinal or foodborne illness-specific surveillance system analogous to the Foodborne Diseases Active Surveillance Network (Foodnet). Only 17 of the 31 major causes of foodborne illness are included as reportable medical events [Reference Scallan6, 7]. AGI is often underreported by traditional surveillance, which only captures cases that seek medical care. Specific diagnosis of foodborne pathogens relies upon laboratory surveillance, leaving many cases undetected [Reference Roy, Scallan and Beach8]. For a reportable medical event to be documented, (1) the ill service member must seek care and (2) submit a stool specimen. The stool specimen is necessary for the laboratory to (3) isolate and identify the organism. The test must yield (4) positive results. Finally (5) the positive result must be entered into the reportable medical events system (Fig. 1). A single omission in this chain of events prevents recording an illness through the current reportable events system.
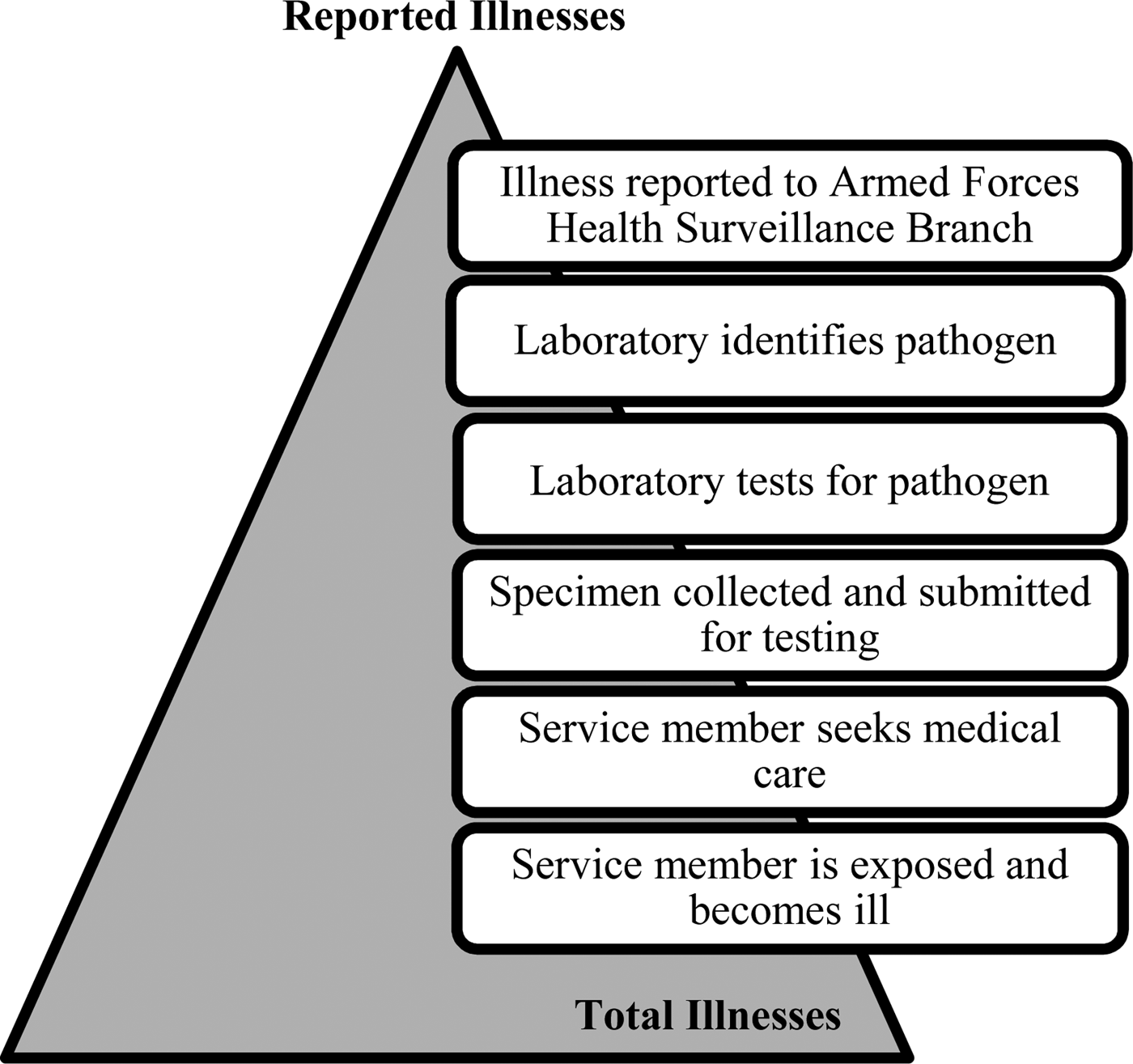
Fig. 1. Burden of Illness pyramid illustrating the steps that must occur for an episode of illness in the active duty Army population to be reported through laboratory surveillance and the reportable medical events system.
This paper purposes to overcome the underreporting and surveillance issues using a web-based survey of the nondeployed active duty US Army population. Scallan et al. demonstrated this technique through the use of telephone surveys, laboratory surveys and data from outbreak investigations [Reference Scallan6].
This paper presents the following study results: the distribution, severity and burden of AGI among nondeployed active duty Army Service Members, AGI risk factor identification and identification of factors impacting the current reportable medical events surveillance system (e.g. service member tendency to seek medical care or provide stool samples). Obtaining these data and estimates are a necessary first step to estimate the burden of AGI caused by specific foodborne pathogens [Reference Roy, Scallan and Beach8].
Ethics statement
The Colorado State University Institutional Review Board (IRB) determined this project qualifies for category 2 exemption from the requirements of the human subject protections regulations as described in 45 CFR 46.101(b) (IRB ID# 131-15H). The US Army Public Health Center (APHC) Public Health Review Board (PHRB) determined that this project did not meet the definition of research as provided by 45 CFR46.102(d) and has approved this project as a Public Health Practice – surveillance (PHRB# 14-316). The purpose of the study was explained to all participants and participation was voluntary.
Methods
Study design and data collection
A cross-sectional electronic questionnaire was distributed via email from 5 April 2015 to 15 May 2015. A geographically representative random sample of the active duty US Army population was selected using a two-stage stratified sampling strategy [Reference Levy and Lemeshow9]. First, the population was divided geographically by regional medical command and then Army installations were geographically and randomly selected in each region. Once the installations were selected, service members were randomly selected using email distribution lists. The required sample size calculation was made using the online application, OpenEpi [Reference Dean and Soe10]. A target sample size of 55 800 was calculated to detect a prevalence of 3%, with 1% precision, using a total population of 528 070 and average military online survey response rate of 2% (N. Thompson, email, 20 January 2015). The number of Soldiers sampled at each installation was proportionally allocated based on the installation population to ensure the equal probability of selection. A total of 61 380 survey instruments were sent via email on 6 April 2015, and reminder emails were sent every 2 weeks until 15 May 2015.
The survey instrument was created using Enterprise Feedback Management (EFM), a web-enabled surveying solution used to capture, analyse, track and act on customer feedback [11]. It contained questions about sociodemographic characteristics, how often respondents ate at various on- and off-post establishments, where certain food items are procured, general health status and any experience of diarrhoea within 30 days of completing the survey. If respondents reported diarrhoea, additional questions about concurrent symptoms, duration of illness, medical care seeking and stool sample submission were asked. The survey questions were developed using a survey provided by Dr Elaine Scallan entitled the FoodNet Proposed AGI Behavioral Risk Factor Surveillance System Survey Module (E. Scallan, email, telephone, 24 July 2014). The survey questionnaire was pre-tested among a group of military personnel. The questionnaire is available from the senior author upon request. Survey results were compiled into an Excel (Microsoft Corporation, Redmond, WA, USA) spreadsheet by APHC staff before being sent to the primary author. No personally identifying information that could link survey responses back to the respondents was included.
Case definition, recall period and inclusion/exclusion criteria
We used the case definition for gastroenteritis proposed by Majowicz et al.: three or more loose stools or any vomiting in a 24-h period, but excluding those (a) with cancer of the bowel, irritable bowel syndrome, Crohn's disease, ulcerative colitis, cystic fibrosis, coeliac disease, or another chronic illness with symptoms of diarrhoea or vomiting, or (b) who report their symptoms were due to drugs, alcohol, or pregnancy [Reference Majowicz12]. Individuals with (a) or (b) were counted as non-cases. Service members who deployed or travelled outside their country of residence within 30 days of completing the survey were excluded. To account for overestimation of the burden of AGI due to the inclusion of primary respiratory cases with secondary gastrointestinal symptoms, we also assessed the frequency and distribution of cases of AGI without concurrent respiratory symptoms [Reference Hall13]. The survey recall period was 30 days prior to the date of the survey response.
Data analyses
Descriptive statistics for categorical variables included frequency, percentages and relative 95% confidence intervals (CI). Differences in proportion were assessed by the χ2test, or Fisher's exact test where appropriate [Reference Baldi and Moore14]. Continuous variables were described by the histogram, mean and standard deviation, or median and range. Differences in diarrhoea duration, vomiting duration, duration of both diarrhoea and vomiting and a number of days of missed work were compared between the five regions using the Kruskall–Wallis test [Reference Baldi and Moore14]. The mean age of respondents was compared between the five regions using one-way ANOVA and Tukey's honest significant difference post-hoc test [Reference Baldi and Moore14].
We used the proportion of respondents with AGI to estimate the 30-day AGI prevalence for the population of interest. (Hereafter, this estimated 30-day prevalence is referred to as prevalence or monthly prevalence.) The point prevalence of AGI was obtained as the proportion of cases with AGI symptoms on the day of filling out the survey. We calculated AGI incidence density in episodes per person-year based on survey responses and used this to estimate the AGI incidence density for the population. (Hereafter, estimated AGI incidence density is referred to as annual incidence). The annual incidence was adjusted to account for those respondents who reported AGI during the 30 day observation and either (a) developed AGI during the 30-day period (incident case), or (b) developed the illness prior to the 30-day period and were still ill at the start of the period, therefore representing existing cases that should be excluded from incidence measures [Reference Majowicz15]. Proportions and annual incidence were adjusted for known demographic differences between those who completed the survey and the target population (active duty US Army Population) by weighting for age, sex, a region of residence, education, rank and race. Gender and age also were weighted by rank and rank was weighted by age [16].
Univariable and multivariable logistic regression were used to identify the factors associated with the occurrence of AGI. In the analysis, the outcome variable was being a case of AGI or not and the explanatory variables were the demographic characteristics of the respondents. Univariable and multivariable logistic regression was also used to construct two healthcare seeking models. Model 1 compared respondents with self-reported AGI who sought medical care with those who did not. Model 2 compared respondents with self-reported AGI who sought medical care and submitted a tool sample to those who sought medical care but did not submit a stool sample. For all regression analysis, independent variables were weighted to compensate for the under- and over-represented demographic factors. (region of residence, age, sex, education, rank and race). Gender and age were also weighted by rank. The models were adjusted to account for the two-stage stratified sampling plan. We assessed independent variables for high correlation with other variables and excluded highly correlated variables from being included in the multivariable analysis.
In the multivariable analysis, the full models included all variables with P-value <0.25 from the univariable analysis. Variables were removed in a step-wise fashion, starting with the highest P-value, until all variables with P-value >0.05 were removed. Independent variables were assessed for confounding by checking a change in model coefficients of ⩾10% as variables were removed/added to the model. Independent variables were assessed for interaction by adding interaction terms back into the model and assessing for significance. The final model fit was assessed using the Pearson's χ 2 goodness-of-fit and deviance test, or the Hosmer Lemeshow Goodness of Fit Test where applicable, with P ⩾ 0.05 indicating goodness of fit.
The salary costs associated with AGI occurrence were calculated using the average hourly base pay for officers and enlisted active duty service members provided by the US Army Research Institute (N. Thompson, email, 26 February 2015).
Descriptive statistics were performed using Microsoft Excel for Mac 2010 (Microsoft Corporation, Redmond, WA, USA), StatCrunch (Pearson Education, 2007–2016) and the online statistical calculator, OpenEpi Version 3.03a 2015 [Reference Dean and Soe10, 17]. Statistical analysis was performed using SAS version 9.3 (SAS Institute Inc., Cary, NC, USA) and StatCrunch (Pearson Education, 2007–2016) [18]. The statistical significance level for these results was <0.05.
Results
Response rate and respondent representativeness
Of the 61 380 emails that were sent, 2.7% received automatic temporary out of office replies, 1.0% received automatic permanent out of office messages (retired, discharged, etc.) and 1.5% were returned as undeliverable. The survey instrument, therefore, reached a total of 60 003 Enterprise email boxes. We were unable to ascertain how many of the emails were opened and read by the recipients vs. being deleted as ‘junk’ emails due to the unknown sender. A total of 2307 surveys were submitted. Of these, 86 were completed by ineligible, non active-duty US Army service members. Twelve of the submitted surveys were completely blank and 162 were less than 50% completed. These responses were excluded from analysis. In total, 2047 completed surveys were received. The simple response rate, not taking into account unread emails, was 3.4%, which is higher than predicted. Typically email response rates for Amy surveys are 2% or less. We believe this is because if an Army service member receives an email from a sender they do not recognise, they delete the email without opening or reading it. Further discussion follows, but we do not believe respondents who chose to answer the survey had a tendency toward the outcome of interest and therefore more likely to respond to the survey. The overall survey completion rate was 92.2%. The demographic characteristics of survey respondents are compared with the 2013 Active Duty US Army demographics in Table 1 [16]. In general, survey respondents were older, more educated, more likely to be female and more likely to be living in Europe than the actual Army population. Respondents who reside in the Europe and Pacific regions tended to be younger on average and those in the Southern region tended to be older on average.
Table 1. Characteristics of respondents, estimates of weighted monthly prevalence (95% confidence interval) and weighted annual incidence rate (95% CI) of self-reported acute gastrointestinal illness (AGI) in the 2015 web-based survey of nondeployed active duty Army service members
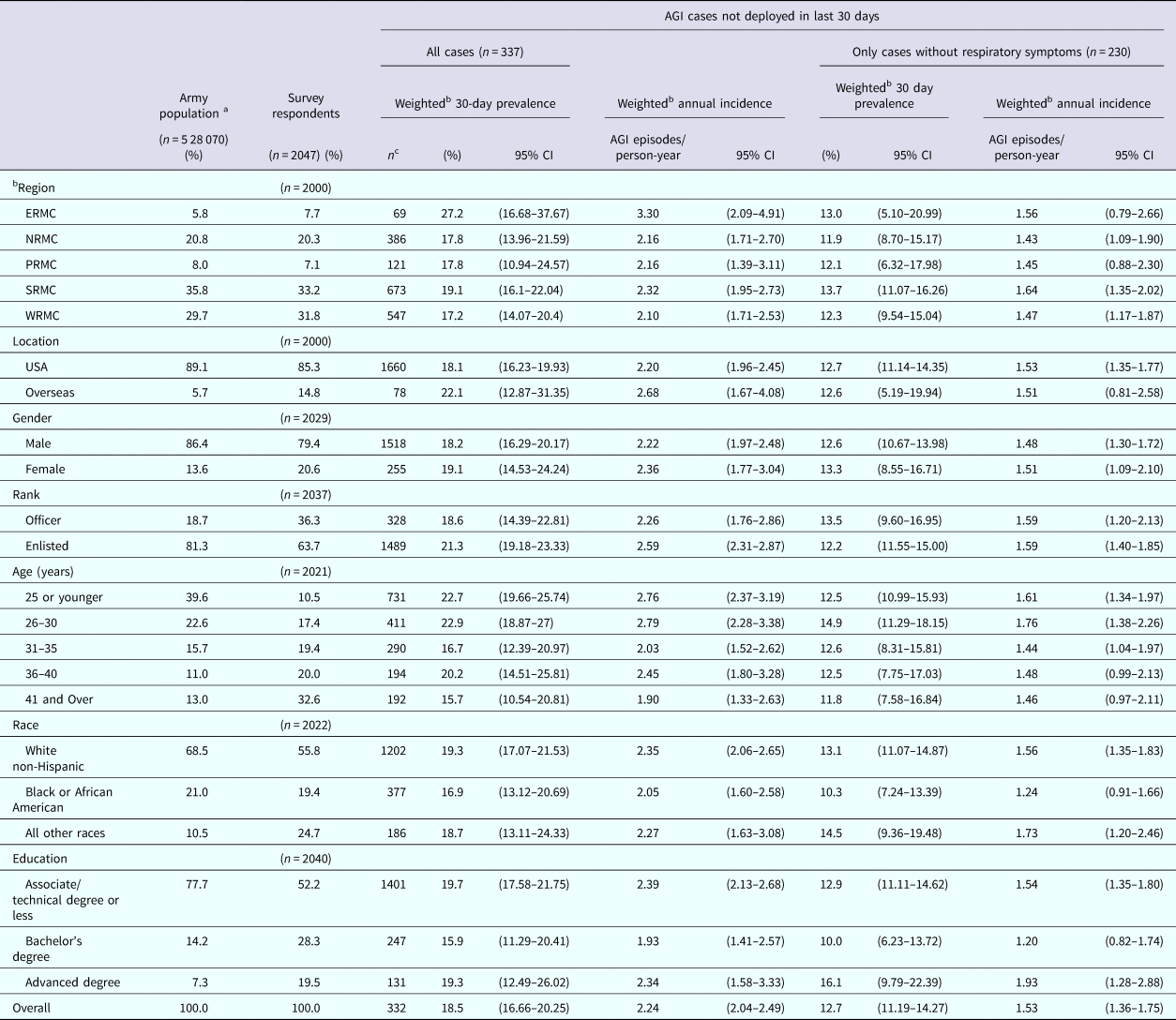
CI, Confidence interval.
ERMC, Europe Regional Medical Command; NRMC, Northern Regional Medical Command; PRMC, Pacific Regional Medical; SRMC, Southeast Regional Medical Command; WRMC, Western Regional Medical Command.
a Data from 2013 Military Demographics Report
b 30-day prevalance and annual incidence rates were adjusted for differences between the survey respondent and US Army population demographics. Gender weighted by rank, rank weighted by gender and age weighted by rank.
c Number at risk are after stratification by region and installation using SAS STRATA statement.
Burden of AGI
Of the 2047 respondents, 739 (36.1%) reported symptoms of gastrointestinal illness in the 30 days prior to completing the survey. Of these, 402 (54.4%) did not meet the case definition of AGI because they reported chronic illness, alcohol, a medication/drugs, or pregnancy as the cause of their symptoms (n = 125), they deployed in the last 30 days (n = 91), or they experienced less than three loose stools and no vomiting in 24 h (n = 186). A total of 241 (11.8%) respondents were excluded from analysis because they deployed or travelled outside their country of residence during the 30 days prior to taking the survey. There were 337 (18.7%) non-excluded respondents who reported experiencing clinical symptoms consistent with the AGI case definition criteria in the 30 days prior to the survey date. Of these, 107 (31.8%) also reported experiencing respiratory symptoms (sore throat, cough) during their illness. The overall weighted monthly prevalence of self-reported AGI was 18.5% (95% CI 16.66–20.25) and the overall incidence rate was 2.24 AGI episodes/person-year (95% CI 2.04–2.49). When excluding cases of AGI that also experienced respiratory symptoms, the monthly prevalence was 12.7% (95% CI 11.19–14.27) and the corresponding incidence rate was 1.53 AGI episodes/person-year (95% CI 1.36–1.75). There were four respondents who reported diarrhoea or vomiting on the day of the survey, corresponding to an AGI point prevalence of 0.22% (95% CI 0.005–0.438). The self-reported AGI episodes/person-year ranged from 2.10 to 3.30 depending on the region where respondents reside. This corresponds to more than 1 075 922 (95% CI 852 047–1 340 801) cases of AGI occurring per year, almost 90 000 cases per month. Enlisted service members who AGI missed an average of 3.67 days of work due to their illness and officers missed an average of 2.61 days of work due to their illness. Using the average base salary, the cost to the government for solely for missed workdays due to AGI is $847 451 629 (95% CI $727 331 502–978 720 151) annually.
Demographic associations
The 30-day prevalence and annual incidence of AGI by demographic characteristics of respondents are reported in Table 1. AGI prevalence and annual incidence rate were highest among those living in the Europe region and the Southern region. Females had a slightly higher AGI prevalence and annual incidence rate than males. The prevalence and annual incidence rate of AGI were higher among enlisted service members than officers. Overall, the AGI prevalence and annual incidence rate was highest among those in the 30 years of age and below categories and lowest among those 41 years of age and older. The annual AGI incidence and 30 day AGI prevalence was highest among white, non-Hispanic individuals and lowest among African Americans. The lowest prevalence and annual incidence rate of AGI were reported among those with a Bachelor's degree. Removing the AGI cases with concurrent respiratory symptoms from the cases decreased the overall prevalence and annual incidence of AGI by 31.4%.
Results of univariable and multivariable logistic regression are reported in Table 2. Risk factors associated with the occurrence of AGI in the univariable analysis included: region of residence, age of respondents, eating at the on-post dining facility (DFAC) and eating at other on-post eating establishments. The variables in the final multivariable model included a region of residence, eating at the DFAC and eating at other on-post establishments when controlling for confounding by gender, rank and race. There was no evidence of confounding or effect modification by the other independent variables. The Pearson goodness-of-fit test P-value was 0.38 and the deviance test P-value was 0.06, indicating good model fit.
Table 2. Association of risk factors with the occurrence of self-reported AGI among nondeployed active duty US Army service members
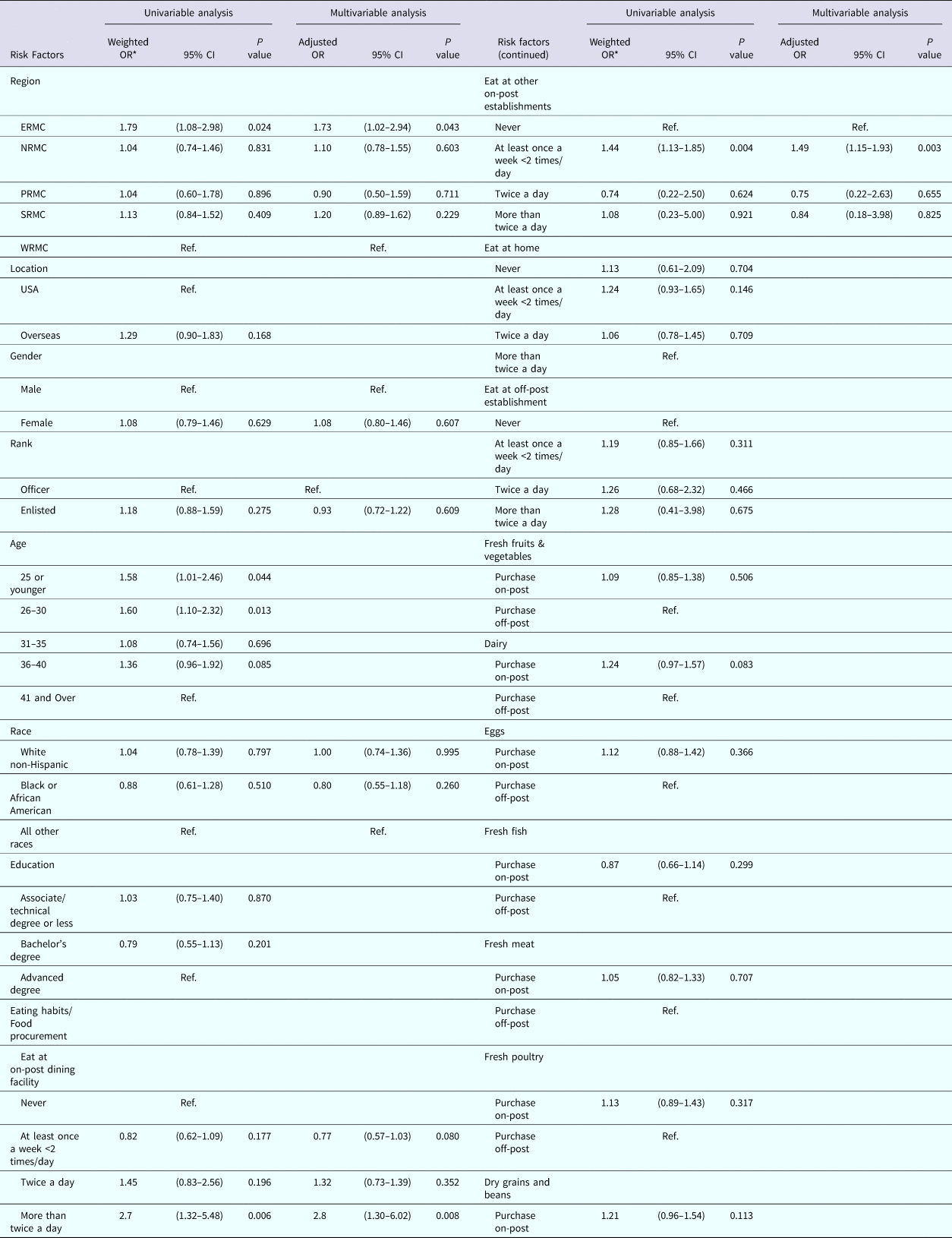
ERMC, Europe Regional Medical Command; NRMC, Northern Regional Medical Command; PRMC, Pacific Regional Medical; SRMC, Southeast Regional Medical Command; WRMC, Western Regional Medical Command.
* Results weighted.
Symptoms, duration, severity of illness and medical care seeking
Characterisation of self-reported AGI cases by the duration of illness, median days of work missed, primary symptoms and care-seeking behaviour of respondents is displayed in Table 3. Of the 337 AGI cases, 233 (69.1%) reported diarrhoea only, 35 (10.4%) reported vomiting only and 69 (20.5%) reported experiencing both symptoms simultaneously. Presence of blood in the stool was reported by 24 (7.1%) cases. The median duration of illness was 2.0 days (range 1–10 days). Median illness duration was longest among those who experienced both vomiting and diarrhoea and diarrhoea only when compared with vomiting only. Approximately 30.9% (n = 104) of ill respondents reported missing work because of their illness for a median length of 2 days (range 1–30) and 20.2% of cases reported seeking medical care for their illness. Of the cases that visited a doctor, 13.2% were asked to submit a stool specimen and 88.9% of those asked to submit a stool specimen did so. Doctors were more likely to ask for a stool sample in cases that experienced both vomiting and diarrhoea (14.3%) than from cases who experienced diarrhoea only (12.9%) or only vomiting (11.1%), though this difference was not statistically significant.
Table 3. Characterisation of self-reported AGI cases by the duration of illness, median days of work missed, primary symptoms and care-seeking behaviour of respondents

Table 4 displays the univariable analyses for Model 1. Factors associated with nondeployed active duty US Army service members seeking medical care for AGI included residing overseas, experiencing more than five loose stools in a 24 h period, having diarrhoea for 3 or more days, experiencing a sore throat or cough, vomiting, vomiting more than five times in 24 h, having both vomiting and diarrhoea for 3 or more days and missing work for their illness.
Table 4. Univariable results for Model 1: factors associated with seeking medical care among nondeployed active duty Army service members with self-reported AGI
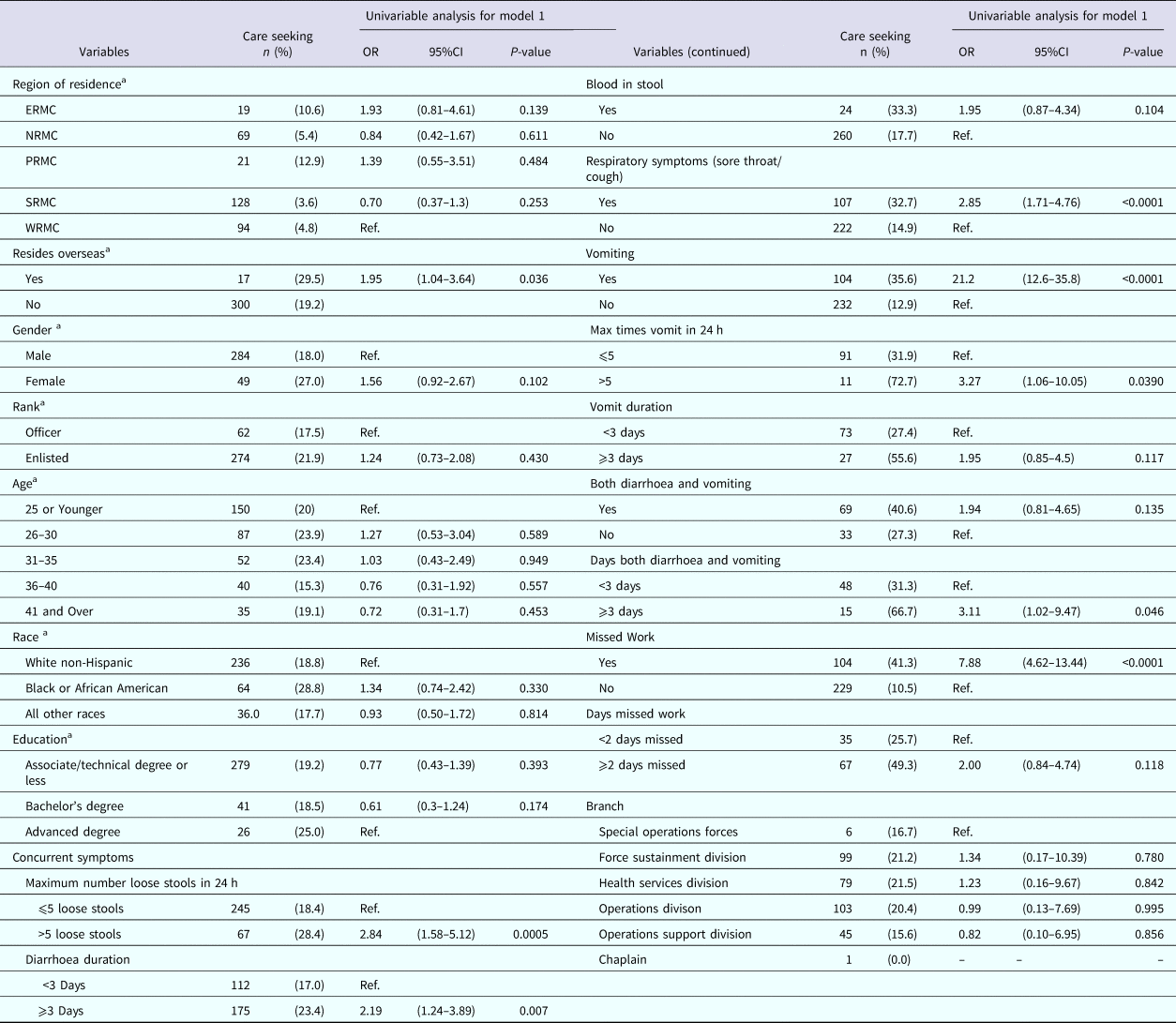
ERMC, Europe Regional Medical Command; NRMC, Northern Regional Medical Command; PRMC, Pacific Regional Medical; SRMC, Southeast Regional Medical Command; WRMC, Western Regional Medical Command.
a Values weighted.
Table 5 displays the multivariable analysis for Model 1. The final variables included: rank, education, experiencing sore throat or cough, vomiting and missing work. There was evidence of multiplicative interaction between missing work and rank. The P value for deviance and Pearson Goodness-of-Fit tests were 0.9356 and 0.9912, respectively, indicating good model fit.
Table 5. Multivariable results for Model 1: factors associated with seeking medical care among nondeployed active duty Army service members with self-reported AGI
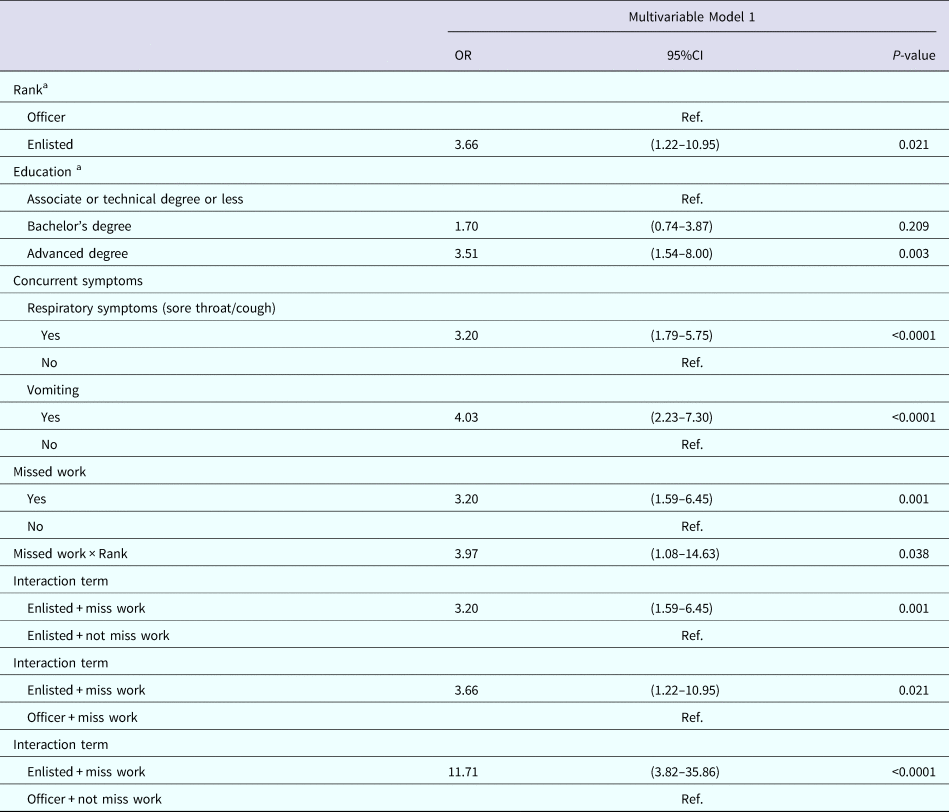
a Results weighted.
Table 6 displays the univariable analyses for Model 2. The only factor associated with active duty US Army service members seeking medical care for AGI and submitting a stool sample in this study was experiencing more than five loose stools in a 24 h period. Though not statistically significant, those who did not experience a sore throat, those who did not experience vomiting, those who did not experience both diarrhoea and vomiting and those who had blood in their stool were more likely to submit a stool sample.
Table 6. Univariable results for Model 2: factors associated with submitting a stool sample among nondeployed active duty Army service members with self-reported AGI
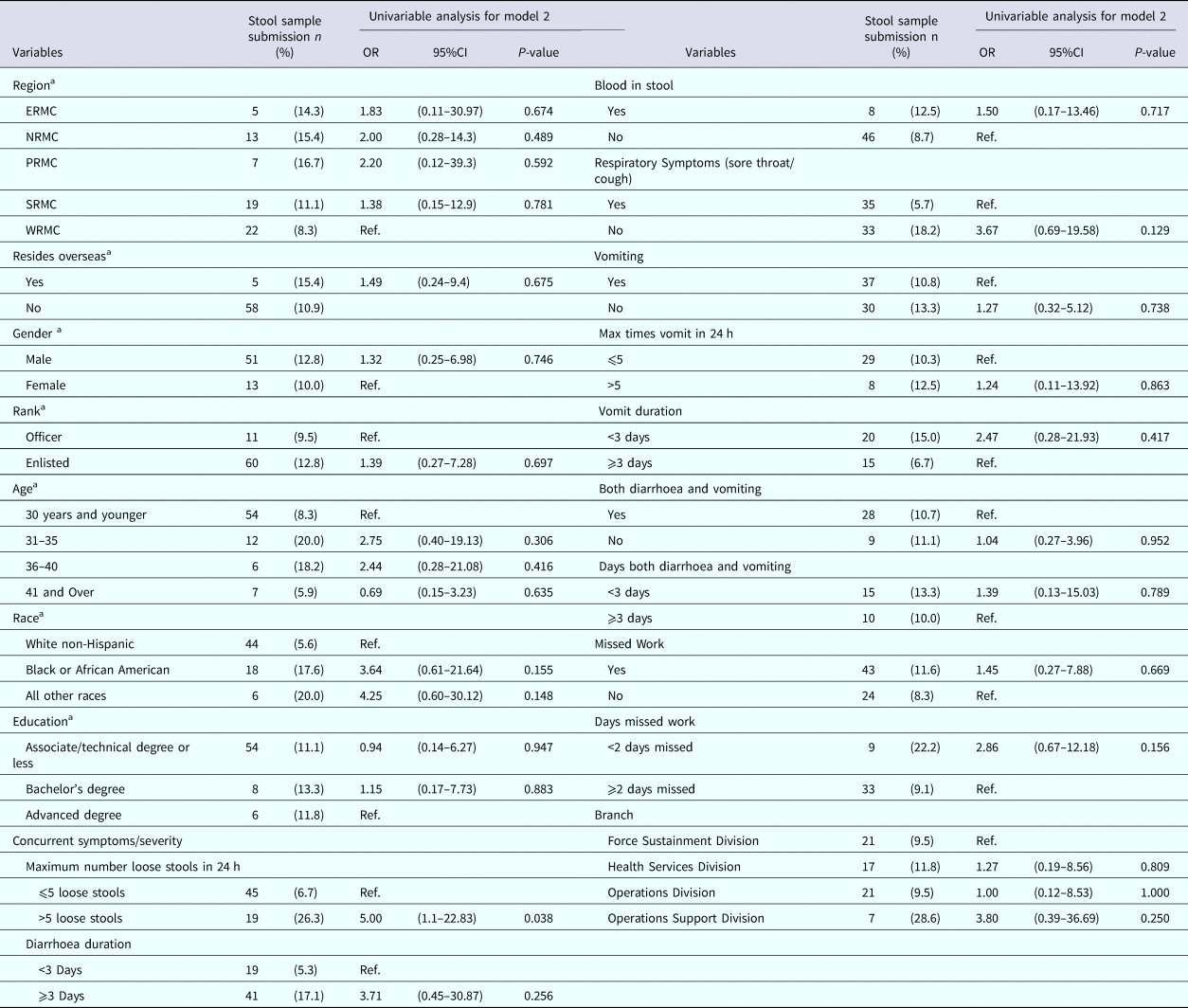
ERMC, Europe Regional Medical Command; PRMC, Pacific Regional Medical Command; NRMC, Northern Regional Medical Command; SRMC, Southeast Regional Medical Command; WRMC, Western Regional Medical Command.
a Results weighted.
Table 7 displays the multivariable analysis for Model 2. The final variables included: experiencing more than five loose stools in a 24-h period and not experiencing a sore throat or cough. Gender and age also were included in the final model to adjust for possible confounding by these variables. The P value Hosmer and Lemeshow Goodness of Fit Test was 0.814, indicating good model fit.
Table 7. Multivariable results for Model 2: factors associated with submitting a stool sample among nondeployed active duty Army service members with self-reported AGI
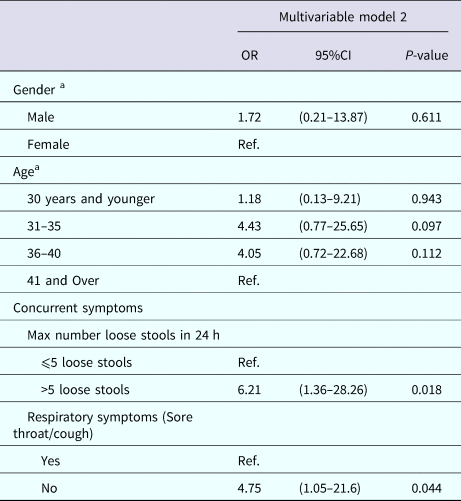
a Results weighted.
Majowicz et al. proposed the minimum set of results that should be reported in AGI studies to facilitate comparison between studies [Reference Majowicz12]. Table 8 displays these results by each regional location, all regions combined and from other similar AGI burden studies for comparison [Reference Majowicz12, Reference Scavia19].
Table 8. Epidemiology of acute gastrointestinal illness under the international case definition (⩾3 loose stool, or any vomiting, in 24 h excluding those (a) with chronic illness with symptoms of diarrhoea or vomiting, or (b) who report their symptoms were due to drugs, alcohol, or pregnancy) in nondeployed active duty Army service members (by regional location and combined), the USA [Reference Majowicz12], Ireland [Reference Majowicz12], Italy [Reference Scavia19], Canada [Reference Majowicz12] and Malta [Reference Majowicz12]
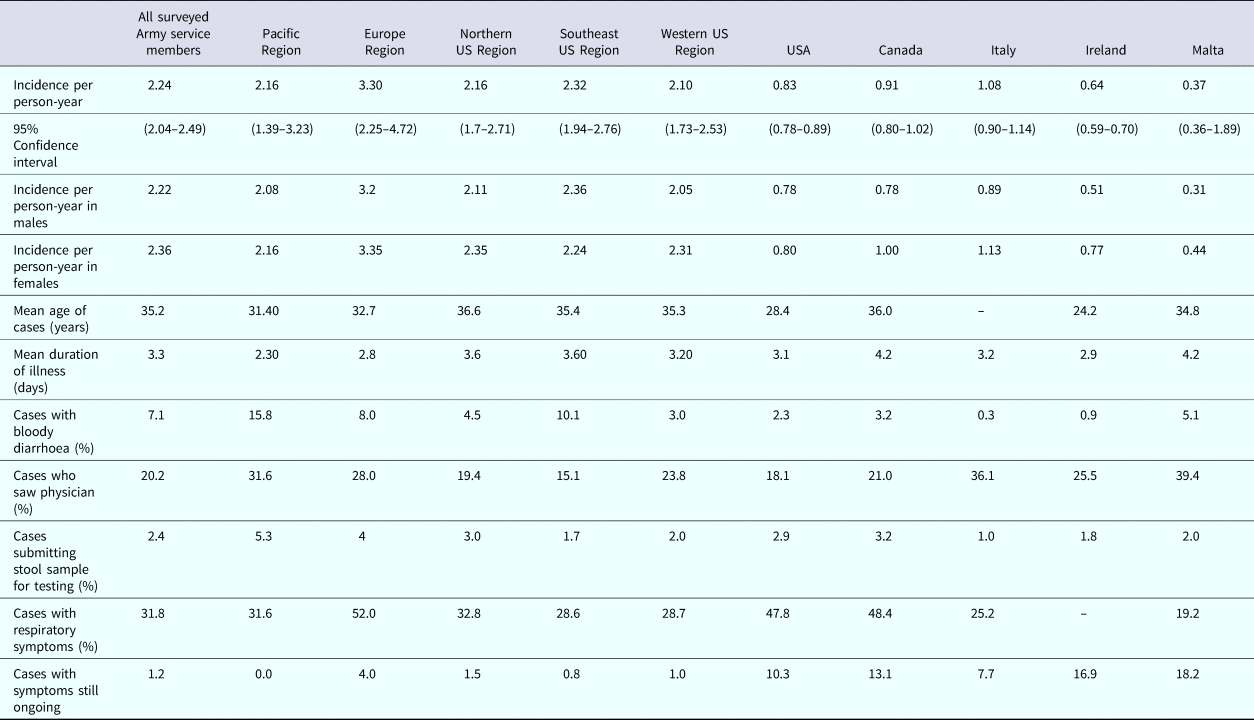
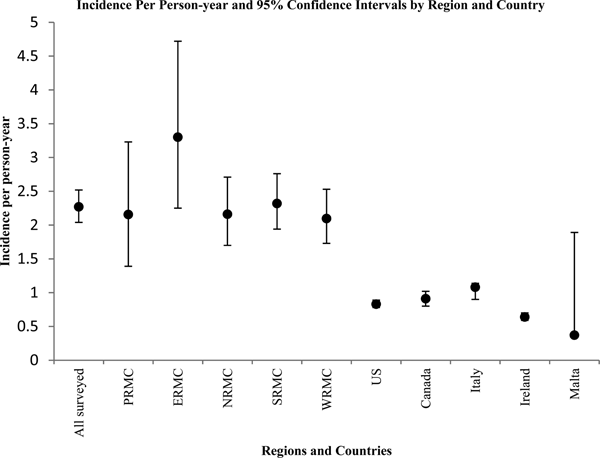
Among the cases reported by US Army service members, cases that reside in the Pacific and Europe regions were younger than those living in the other regions. Those residing in the Pacific region reported blood in their stool more often than those living in the other four regions and this difference was significantly greater than those living in the Western US region. Service members with AGI who reside in the Pacific region also were more likely to visit a physician for their illness, though this difference was not statistically significant. Physicians in the Pacific region were more likely to submit stool samples for testing than in the other regions, but this difference was not statistically significant. Those residing in the Europe region reported concurrent respiratory symptoms significantly more often than those living in the four other regions combined. When considering all self-reported AGI cases among nondeployed active duty US Army personnel who responded to the survey, the average age of cases (35.2 years) were of similar age to cases in Canada (36.0 years), but older than all other countries listed in Table 8. US Army service members reported blood in their stool more often than all five-comparison countries but sought medical care less than cases in Canada, Italy, Ireland and Malta. The median duration of illness among US Army service members was less than the mean duration of illness reported by all other countries. The proportion of cases with respiratory symptoms was less than that reported in the USA and Canada. The number of cases with illness on the date of interview/filling out the survey was less than all other comparable countries.
Figure 2 displays the corresponding incidence per person-year and 95% confidence intervals in a graphical format. Overall, the annual incidence per person-year was significantly higher among surveyed US Army service members than in the USA Canada, Italy and Ireland (confidence intervals do not overlap).
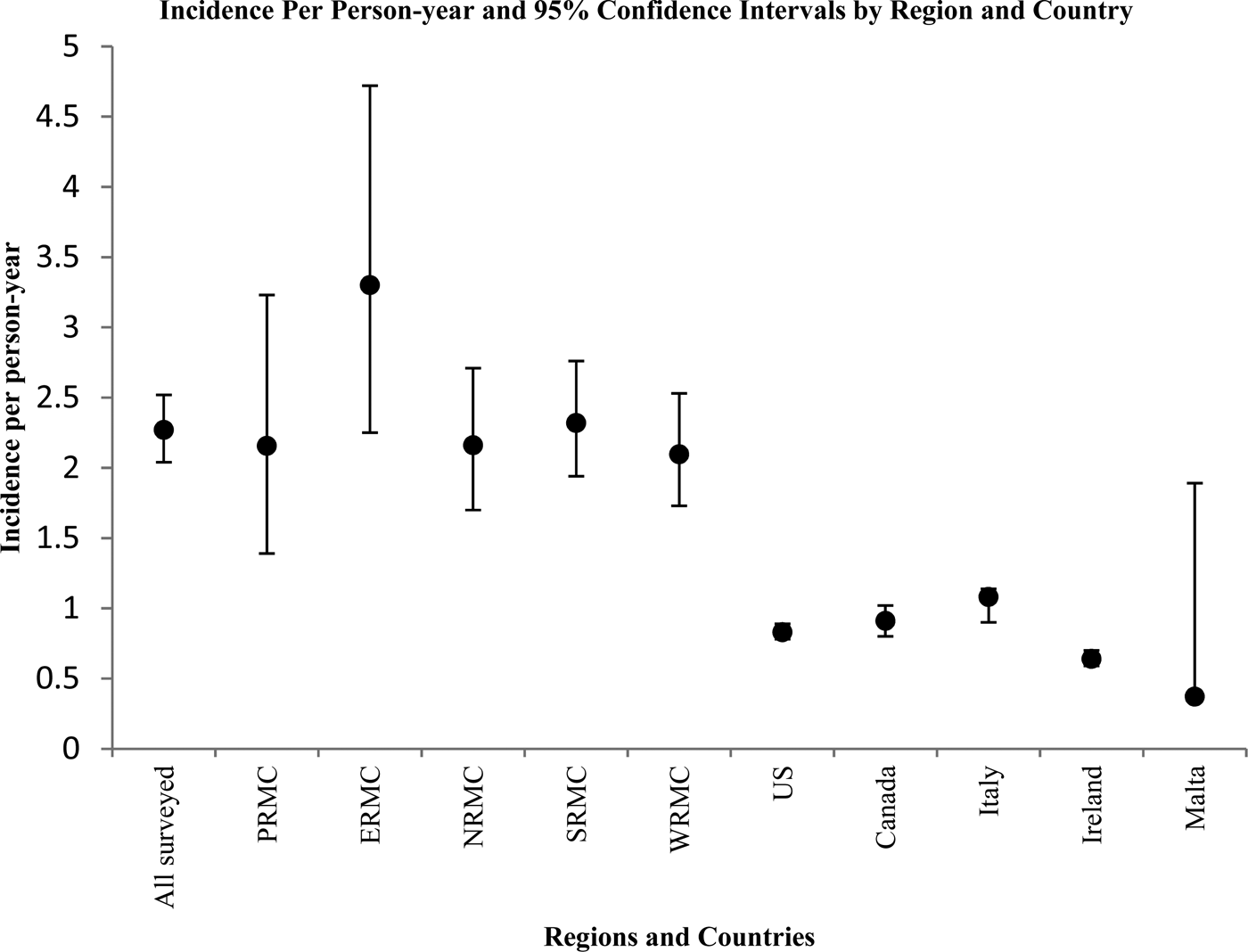
Fig. 2. Graphical representation of point estimates and 95% confidence intervals for incidence of AGI episodes per person-year by region and country.
Discussion
To our knowledge, this is the first worldwide survey conducted in the active duty US Army population with the objective of describing the distribution, severity and burden of AGI, identify risk factors for AGI and identify factors associated with seeking medical care and submitting a stool sample for AGI in this unique population. The overall estimated annual incidence AGI of 2.24 episodes per person-year (95% CI 2.02–2.49) was much higher than estimates reported by studies in developed countries using the same case definition [Reference Scavia19–Reference Wilking26]. The rate of AGI is alarmingly high in this study, especially considering the age groups most often associated with higher incidence of AGI are excluded due to military age restrictions [Reference Herikstad27–Reference Hoogenboom-Verdegaal30].
Survey respondents with self-reported AGI may also report concurrent respiratory symptoms. These symptoms may be due to primary respiratory infections, primary gastrointestinal infections, or both [Reference Hall13]. Of the respondents that met the case definition for AGI, 31.8% also reported experiencing respiratory symptoms (sore throat, cough) during their illness. This percentage is less than seen in similar studies in the USA (46.7%) and Canada (41.8%) and comparable with that seen in Australia (28.6%) [Reference Hall13]. When excluding cases of AGI with concurrent respiratory symptoms, the overall average incidence rate decreased to 1.55 AGI episodes per person-year, a 31.7% reduction. This reduction is similar to the reductions seen in studies conducted in Australia (30% decrease) and less than reductions seen in the USA (>50% decrease) and Canada (40%) [Reference Hall13]. The largest reduction in the incidence of AGI after excluding cases with respiratory symptoms was seen in the European region.
Risk factors for AGI identified in this study included living in the Europe region, eating at the DFAC on average more than twice a day and eating at other on-post establishments at least once a week, but less than twice per day. Similar AGI burden studies in Europe describe annual AGI incidence rates consistent with other developed countries but much lower than in our population [Reference Scavia19, Reference Muller, Korsgaard and Ethelberg24, Reference Gauci31–Reference Kuusi34], so it is unlikely that living in Europe is the only factor contributing to the increased AGI in this population. Eating at higher risk establishments, or procuring food from unfamiliar, local sources could be contributing factors. The association could also be unrelated to the consumption of contaminated food and should be investigated further.
The association between eating at the DFAC and other on-post food establishments and the occurrence of AGI could be due to a breakdown anywhere in the food protection program, including unsanitary conditions at these establishments, poor food worker education and hygiene, procurement of unsafe food products, improper storage of food on-post, improper/insufficient inspection procedures and mishandling of food taken out of the DFAC by consumers. Preventive medicine and veterinary services should perform thorough inspections of all on-post dining facilities to determine possible causes for the identified increased risk of AGI among those who eat at these facilities.
Other similar studies found an increased tendency for women to develop AGI, citing increased food handling [Reference Majowicz15, Reference Sargeant, Majowicz and Snelgrove25, Reference Gauci31] and caring for their children [Reference Gauci31, Reference Scallan35, Reference Thomas36] as potential causes. In our study population, females did not have a statistically increased propensity for developing AGI. This could be due to female active duty US Army service members spending less time in the home preparing meals and caring for children. Our findings were consistent with similar studies conducted in Cuba [Reference Aguiar Prieto37], Malta [Reference Gauci31] and Denmark [Reference Muller, Korsgaard and Ethelberg24] that cite cultural practices or study bias for their results.
If our estimates are an accurate reflection of the true incidence of AGI among active-duty nondeployed US Army service members, additional studies to determine more risk factors for AGI in the US Army are warranted so we can develop policies and intervention strategies to reduce AGI. The exceedingly high AGI incidence rates we found should be considered in future burden of illness studies in the USA and overseas, as they may influence the outcome.
Bloody diarrhoea and duration of illness are indicators of AGI severity [Reference Scallan38]. In general, compared with other studies, our cases were more severe, were shorter in duration and had fewer reports of concurrent respiratory symptoms (Table 8). This could mean that our survey did a good job of capturing both the severe and mild primary gastrointestinal disease. This could be a reason why our reported AGI incidence rate is so much higher than other studies. Our respondents sought care more often than cases in the USA. This could be due to increased access to care. All active duty service members have access to free healthcare either on the installation where they are stationed or through the military's medical insurance if stationed remotely. Canada, Italy and Ireland also have National healthcare services and may explain why care seeking is higher in these countries. Though stool samples were only requested in 13.2% of cases, almost all of those who were requested to submit a stool sample did (88.9%). This means educating physicians regarding the importance of collecting a stool sample for a definitive diagnosis of illness caused by foodborne pathogens may result in increased stool sample submission and improve detection by laboratory-based surveillance.
Similar studies report blood in the stool as a reason for seeking medical care more frequently [Reference Scallan38–Reference Mead40]. However, in our study, which had a higher proportion of bloody stools than other studies, blood in the stool was not associated with seeking medical care in the multivariable models, regardless of the case definition. Our surveyed population was more likely to seek medical care if they experienced the clinical symptoms of vomiting and a sore throat and/or cough. Noroviruses are the leading causes AGI among people seeking medical care and a common clinical sign of Norovirus is vomiting (which can lead to a sore throat) but non-bloody diarrhoea [Reference Hall41]. It is possible that our study captured cases of Norovirus more than other causes of AGI that result in care seeking.
Duration of illness also was cited often as a factor associated with seeking medical care [Reference Scavia19, Reference Scallan38]. We found this to be the case during univariable analysis, but not during multivariable analysis. We repeated the analyses excluding cases that experienced sore throat or cough to see if additional risk factors for AGI could be identified. After excluding these cases, there was not enough power to detect any significant associations. Other published symptoms associated with seeking medical care, but not investigated in our study due to inadvertent oversight, include abdominal cramps, fever and nausea [Reference Scallan38].
Factors associated with seeking medical care and submitting a stool sample included having more than five loose stools in 24 h and absence of a sore throat or cough. This information helps us to determine what symptoms guide a physician's decision about whether or not to request a stool sample. In general, it appears that physicians do not request stool specimens from AGI cases that may be caused by primary respiratory illness. The frequency of diarrhoea appears to be the biggest driving factor for physicians to request a stool sample from our population.
This study helps us to determine the difference between AGI cases detected by surveillance and AGI cases not detected by surveillance. Our survey may over-represent cases with primary diarrhoeal illness while underestimating cases with bloody diarrhoea. According to our results, US Army service members with AGI (bloody) are less likely to seek care and submit a stool sample than that of the general US population.
As with any study, our estimates may have been biased by a number of factors. To our knowledge, this is the first population-based AGI burden study to use a web-based survey for data collection. Problems with web-based surveys in general center around the fact that a limited percent of the population has access to the internet and creating sampling frames that give complete coverage of the general population of interest are very difficult, if not impossible [Reference Tourangeau42]. The military population is unique in that all service members are assigned an email address and have access to the Internet. Because of this, we were able to randomly select US Army service members based on assigned installations.
Even though our response rate was higher than in previous online surveys of the military (N. Thompson, email, 20 January 2015), relative to population-based telephone surveys conducted in other countries, our response rate was extremely low. Though recent studies have demonstrated little to no relationship between nonresponse rates and nonresponse bias, the ‘low’ response rates in these studies (36.0%) was much higher than our simple response rate of 3.4%, so nonresponse bias is certainly a concern in our study [Reference Tourangeau42]. However, as stated previously, we do not know how many individuals deleted the email without even opening it because it was from an unknown sender. We have no reason to believe these individuals would have any tendency towards or away from the outcome of interest. If those who opened the email and chose not to respond (non-responders) were more or less likely to have experienced AGI in the previous 30 days, our results would be biased in favour of those who did respond. If those who experienced AGI in the previous 30 days felt it was important to answer the survey (more so than those who did not), they would be over-represented. To avoid this bias, we did attempt to structure the email and the questionnaire in a way that would not lead the survey recipients to believe the survey was about their experience with AGI. The initial survey questions were about where service members procure food items and how often they eat at on-post establishments. Questions about experience with AGI did not come until about halfway through the survey. Also, an individual may be more willing to answer questions about their experience with unpleasant events such as diarrhoea, vomiting and bloody stool when answering a web-based survey, as opposed to a telephone or face-to-face interview with a live person. Web surveys may, therefore, result in a more accurate reflection of the burden of diarrhoeal illness. Of the individuals that started the survey, 92.2% completed the survey, so very few non-responders opened the survey and chose to quit before completing the survey. We feel strongly that our results are no more or less biased than any other similar study.
Certain factors may have led to an underestimation of AGI incidence as well. Due to time constraints and workload on respondents, we were only able to launch the survey once, so we cannot adjust results based on seasonal variation. This survey was launched on 6 April 2015, and respondents had until 15 May 2015 to respond. 868 surveys were completed by 15 April, an additional 510 by 30 April and the remaining 669 by the close of the survey, so the self-reported cases could have occurred anywhere from 6 March to 15 May. In general, rates of AGI are highest in winter months and lower in the spring, so our survey may even further underestimate the incidence of AGI. We also used a 30-day recall period for this study. A US study showed that recall period length has an impact on estimates of the prevalence of AGI [Reference Cantwell43]. They found that annual rates of AGI estimated using a 7-day recall period were 1.8–3.4 times higher than when using a 1-month recall period [Reference Cantwell43]. Another study showed the opposite results, finding that a 3-week recall period incidence was almost three times higher than the rate estimated through active surveillance [Reference Wheeler44]. It is difficult to assess the impact our recall period had on calculated AGI prevalence and incidence data. Conducting another survey using more than one recall period, or a prospective study could help to increase the accuracy of these estimates.
We note that physician stool sample request and submission is low compared with other studies. Though other studies use similar approaches, one of our identified limitations is that our data are based on patient self-report and not a medical record review of stool sample submission tendencies.
Even though different sources of bias could have limited the accuracy of our burden estimates, this is study is important to understand more about the true burden, distribution, severity, risk factors and care-seeking behaviours for AGI in the active duty Army population. In addition, the data can be used in estimating the burden of illness by preventable enteric pathogens, such as foodborne pathogens. Regardless, we determined Army service members care-seeking behaviours, AGI risk factors, and stool sample submission rates are different than the general population, so when estimating burden of AGI caused by specific foodborne pathogens using methods like Scallan et al. unique multipliers must be used for this subset of the population [Reference Scallan6]. The study legitimises not only the importance of AGI in the active duty Army population but also highlights opportunities for public health leaders to engage in simple strategies to better capture AGI impact so more modern intervention strategies can be implemented to reduce burden and indirectly improve operational readiness across the Enterprise.
Disclaimer
The views expressed in this article are those of the authors and do not reflect the official policy or position of the US Army Public Health Center, US Army Medical Department, Department of the Army, DoD, or the US Government.
Acknowledgements
We thank Dr. Rebecca I. Evans (COL, US Army Veterinary Corps) for serving as the administrative and technical support lead at the Army Public Health Command, Mr. Chris Weir (Army Public Health Center) for programming multiple iterations of the survey questions into the online survey tool, Dr. Roy N. Emanuel II (Johns Hopkins Applied Physics Lab) and Mrs. Mary Lou Emanuel for proofreading and final comments on the manuscript, Dr. Elaine Scallan (University of Colorado Denver) for providing her expertise and support throughout the entire project, and Dr. Thomas E. Honadel (COL, US Army Veterinary Corps) for his unwavering support, mentorship and guidance.
Author ORCIDs
S. B. Mullaney 0000-0003-0948-012X.