INTRODUCTION
Infection with the spirochaete Borrelia burgdorferi (Lyme borreliosis) is the most common human tick-borne zoonosis in the UK and Europe [1–Reference Dillon, O'Connell and Wright7]. Detailed and accurate recent data on the prevalence and distribution of Lyme within the UK is sparse.
The vectors are a number of closely related hard-bodied ixodid ticks (Ixodes ricinus complex). Commonly known as ‘sheep ticks’, or ‘deer ticks’, the feeding hosts include many species of wild and domesticated mammals, birds and some reptiles. Humans may be incidental hosts at any stage of the Ixodes life-cycle [2, Reference Stanek5, Reference O'Connell6].
UK national enhanced surveillance data demonstrate increases in the annual incidence of laboratory diagnoses of Lyme borreliosis from 268 cases in 2001 to 972 cases confirmed in 2011 [1]. Many cases are treated clinically in primary care without serological testing, so these figures are accepted to under-represent the true incidence. However, since October 2010 under the Health Protection (Notification) Regulations 2010, every microbiology laboratory (including those in the private sector) in England is required to notify all laboratory diagnoses of borreliosis to the Health Protection Agency (now Public Health England; PHE). Previously, reporting by laboratories was on a voluntary basis. The laboratory-diagnosed cases reported to PHE give a national incidence of about 1·7 cases/100 000 population [1].
The clinical presentation, diagnosis and management of Lyme disease is well established [2, Reference Mygland4–Reference Wormser8]. Lyme borreliosis may be asymptomatic and seropositivity varies across Europe from 2% to 5% although this figure may be higher in risk occupations such as forestry workers [Reference Dehnert9]. The clinical manifestations of Lyme disease are multi-systemic and may present with early localized [Reference Duncan, Carle and Seaton10], early disseminated, or late disease [Reference Stanek5, Reference O'Connell6]. Lyme borreliosis is prevalent in the temperate zones of North America, Europe and Asia, with sporadic cases elsewhere. Much of the published epidemiological data reflects disease patterns in the USA where B. burgdorferi sensu stricto is the major pathogenic species [Reference Wormser8]. Within the UK and Europe, Borrelia garinii and Borrelia afzelii are responsible for most Lyme disease although other genospecies can cause clinical disease [Reference Mygland4–Reference O'Connell6, Reference Wilske11]. Collectively, these genospecies are classified as B. burgdorferi sensu lato. As a result of these differences in infecting genospecies, the natural history and clinical manifestations of Lyme disease differ in Europe compared to North America [Reference Stanek5, Reference O'Connell6, Reference Wormser8].
The prevalence and variability of serological changes secondary to Lyme disease, the variable multi-systemic clinical manifestations and the presence of late complications has led to debate as to the possibility of ‘chronic Lyme disease’. This debate has broadened to include the possibility of ‘seronegative’ Lyme disease. A variety of post-infectious conditions, fibromyalgia, chronic fatigue, psychiatric illness and even neurological conditions such as multiple sclerosis, motor neurone disease, atrophic lateral sclerosis, vascular neurological damage have been attributed to borrelial infection. These cases of genuinely symptomatic patients represent a significant body of people and as a result a number of patients’ advocacy groups have developed [12–14]. A treatable infective aetiology is source of hope but there is an absence of clear case definition or diagnostic criteria and hence a dearth of evidence-based therapeutic trials [13, 14]. These ‘seronegative Lyme disease’ patients tend not to have significantly positive Borrelia serology or are seronegative by currently used conventional, validated diagnostic tests. They probably reflect a range of different aetiologies, which include the possibility that they may have Borrelia infection that has not mounted an antibody response, that they may be infected by an as yet undiscovered strain of Borrelia or a different organism altogether, or that their symptoms are due to a non-infective cause. Ticks are known to transmit other pathogens such as rickettsias [Reference Masters15] and other strains of Borrelia, as in Southern Tick-Associated Rash Illness (STARI) caused by Borrelia lonestari [Reference Due16]. It is evident that there are several uncertainties in causative agent, definition, diagnosis and treatment of Lyme disease-like illnesses which require further research.
This paper reports data on the presentation of serologically confirmed cases of Lyme disease presenting to an infection clinic in the microbiology department at an acute hospital in the UK over two decades, and also discusses the phenomenon of so-called ‘chronic Lyme disease’.
METHODS
The Royal Hampshire County Hospital (RHCH) in Winchester serves a population of about 250 000 that live in the city of Winchester, surrounding country towns and a large rural area of central and southern Hampshire. It does not include the New Forest, an area traditionally associated with Lyme disease, but it does include the Test and Itchen valleys and the Harewood Forest near Andover.
The aim was to capture all cases of Lyme disease over this period in this population by clinical and laboratory surveillance. As a mechanism of increasing local medical awareness of Lyme disease, local infection surveillance feedback had been distributed via a newsletter since 1991 and local general practitioners (GPs) were encouraged to refer patients with suspected Lyme to the infection clinic at the hospital or failing that to discuss cases by telephone with an infection specialist. This aimed to pick up all cases of Lyme disease who presented to GPs in the area.
A case definition of Lyme disease for the purpose of this series was a patient who presented with clinical features of Lyme disease – dermatological, neurological, rheumatological or cardiac, but who also demonstrated positive serology by recognized diagnostic tests in the UK and confirmed by the UK Lyme national reference laboratory. This was a two-tiered diagnostic system involving the two immunodiagnostic methods of ELISA and immunoblotting. Cases included in the series presented to the infection clinic at RHCH between 1992 and 2012 as outpatients or inpatients in the hospital or as a result of a telephone consultation with a GP within the hospital catchment area. This number has been used to calculate the incidence of the disease locally. Although it is believed that this combination of clinical and laboratory surveillance would have captured most cases, a small number may have been missed, as with increasing awareness it is likely that some cases of primary Lyme were being treated empirically without serological diagnosis or consultation with an infection specialist.
Serological tests have changed over the last 21 years; however, the purpose of this paper is not to report the efficacy of serological testing. Initially all tests were performed at the Lyme Disease Reference Laboratory in the Southampton Public Health Laboratory, subsequently the Health Protection Agency and most recently at RIPL, PHE by an ELISA technique and confirmed by IgM and IgG Immunoblot tests. From 2004 screening ELISA tests were performed using the VIDAS bioMérieux technique (total antibody and latterly specific IgM and IgG), with positive samples referred to the reference laboratory for further testing. Cases which screened negative, but were clinically suggestive were also referred to the reference laboratory and in these cases a later serum sample was also sought from the patient.
Demographic data and clinical presentation were recorded from patients with serologically confirmed Lyme disease on a clinical record data base. This data included age, sex, history of a tick bite, source and date of the tick bite, date of first symptoms, history of symptom – dermatological, neurological, rheumatological or cardiac, date of presentation, serology results, treatment and outcome. Patients were offered access to the clinic after treatment should they require it or if they were concerned about recurrence or relapse.
Many clinical referrals of patients with ‘suspected’ Lyme disease, ‘chronic’ Lyme disease or ‘seronegative’ Lyme disease have also been received over this period. These patients probably represent a mixed variety of conditions of as yet unknown aetiology and pathogenesis and although they now represent a greater clinical challenge in terms of patient numbers than serologically confirmed Lyme cases, they are not included in this paper because there is no clear case definition or diagnostic test. The clinical condition is discussed later and a case definition and name is proposed for this syndrome. Such patients with so-called ‘chronic Lyme disease’ were excluded from the clinical case series, as the diagnosis could not be verified by validated laboratory diagnosis and chronic symptoms were subjective.
RESULTS
Five hundred and eight clinical cases of Lyme disease were diagnosed and serologically confirmed between 1992 and 2012. This represents a mean annual rate of 9·68/100 000 population (range 1·6–18·4) (Fig. 1), almost 10 times the reported national rate [1].

Fig. 1. Rate of Lyme disease per 100 000 population in the Winchester area.
Of these cases, 239/508 (47%) were male with a bimodal age distribution. Peaks occurred during the first and sixth decades of life (Fig. 2). One hundred and ninety-one out of 508 (38%) patients described a preceding bite although a tick was not always identified. One hundred and sixty-seven (33%) patients gave a clear negative history of a bite and 150 (29%) were not sure but possibly bitten. Of those patients who recalled a bite, 26 (5·1%) described being bitten while abroad (Europe, n = 20; North America, n = 5; Central America, n = 1). The majority of cases were acquired in Hampshire and others elsewhere in the UK – Wiltshire, Sussex, Dorset, Devon and Scotland. Within Hampshire, bite locations were widely distributed throughout the county and not confined to the New Forest, with a particular hotspot being the semi-rural area of Chandlers Ford where frequently tick bites occurred in gardens of the patient's own home.
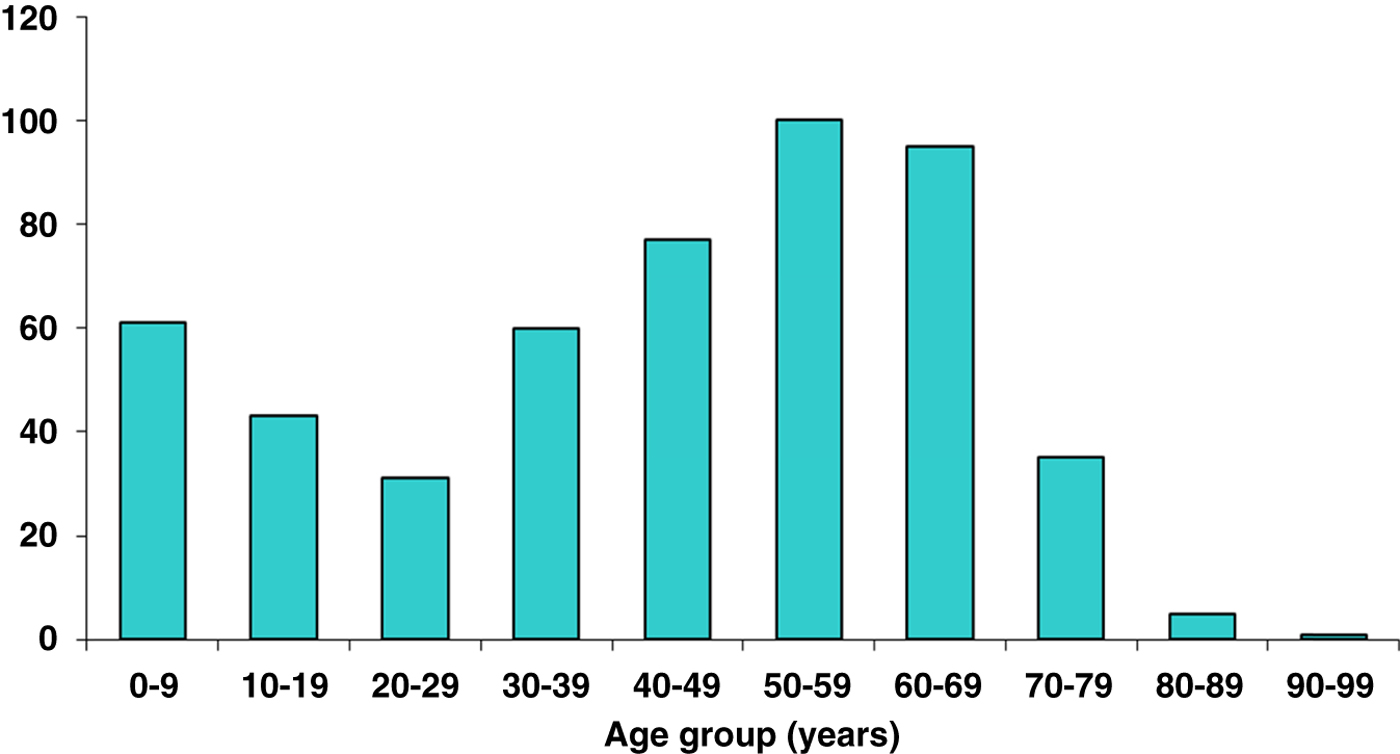
Fig. 2. Lyme disease by age.
Presentation demonstrated a significant seasonal variation, favouring the UK summer months of June–August. Data on time of tick bite (n = 191) and time of first clinical symptom (n = 415) followed the same seasonal distribution, but the most common months for tick bites were reported as May–July with first symptoms presenting June–August (Fig. 3). Median time from tick bite to first symptom was 15 days [interquartile range (IQR) 9–28 days]. The median time from first symptom to diagnosis was 14 days (IQR 2–31 days).

Fig. 3. Month of tick bite, first symptom and confirmation of diagnosis.
The first symptoms described by the patients are given in Table 1. Of these patients, 97 (20%) described ‘flu-like’ symptoms of short and limited duration, usually alongside more specific dermatological or neurological symptoms. These included ‘flu-like illness’, muscle and/or joint aches, tiredness, lethargy, sleep disturbance, depression or feeling low. Most patients reported these symptoms resolved rapidly on antibiotic treatment. Even patients with more longstanding neuroborreliosis generally reported rapid resolution of non-specific symptoms once antibiotics were started.
Table 1. First symptoms described by the patient in this series
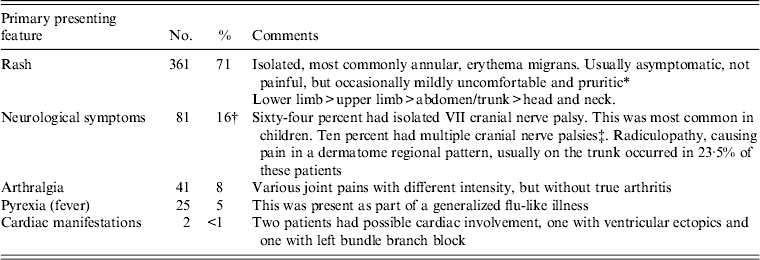
* In the minority of cases it was frankly cellulitic in appearance, showing intense erythema and swelling (this may due to an element of secondary bacterial infection), but no discharge and little discomfort in comparison to the apparent degree of inflammation.
† Two patients had meningo-encephalitis requiring admission to hospital. In these patients CSF was collected and demonstrated slightly raised CSF protein (680 and 840 mg/l; normal limit <450 mg/l), CSF lymphocytosis (32 and 75 lymphocytes × 10/l; normal <5), and the presence of Borrelia antibodies in the CSF. One patient, previously reported [Reference Klempner28], had myelitis (Figs 4, 5), presenting as specific fine motor alteration of the upper limbs and generalized neurological symptoms of lethargy and depression. He had a cordal lesion between C4 and C9 visible on MRI (Fig. 4). CSF was unfortunately not collected from this patient.
‡ In children a VII cranial nerve palsy often followed a bite within the hair line where the rash was unnoticed until careful examination. Two patients with later diagnosed neuroborreliosis described a rash which resolved spontaneously some weeks before, although one of these in the groin had been treated with topical fluconazole.
One patient with Lyme disease was pregnant, presented with erythema migrans and was serologically confirmed at 16 weeks. This patient was treated in pregnancy with amoxicillin, had a normal pregnancy and delivery with no sequelae in the baby.
A notable feature of Lyme disease was that routine haematology and biochemistry tests were rarely abnormal. The peripheral white blood cell count was usually normal. The C-reactive protein was usually normal or marginally raised, <15 mg/l.
Treatment
Patients in this series were treated according to standard currently published guidelines [2]. Data on treatment were recorded for 481/508 patients. Most patients were treated with oral antibiotics as outpatients: 2 weeks with primary dermatological Lyme disease, 3 weeks if there was a delay in diagnosis, systemic symptoms or minor neurological signs such as VII cranial nerve palsy or radiculopathy. A minority of patients received longer courses and the indication for this was more complex neuroborreliosis. The longest course was for 3 months of doxycycline in the case of myelitis until the cordal inflammation had resolved (Figs 4, 5). The majority of patients (344/481, 72%) received tetracyclines (doxycycline, oxytetracycline). One hundred and twenty-eight (27%) patients received penicillins (amoxicillin, phenoxymethyl penicillin), three received macrolides [erythromycin (n = 1), resulting in a relapse requiring doxycycline treatment; clarithromycin (n = 2)]. Nine patients with complex neuroborreliosis were initially admitted to hospital and treated with intravenous ceftriaxone [meningoencephalitis (n = 2), cranial nerve palsy (n = 2), myelitis (n = 1), radiculopathy/peripheral nerve palsy (n = 4)]. Intravenous ceftriaxone was administered for between 5 and 7 days before switching to oral doxycycline.

Fig. 4. Lyme myelitis. Cordal lesion C4–C9 before treatment.

Fig. 5. Lyme myelitis after 3 months of antibiotics.
Recurrence
Seven (1·4%) patients in the course of the 21 years required a late follow up (>30 days post-treatment) consultation because of concerns about recurrence or re-infection. In all cases this occurred beyond 1 year after diagnosis and treatment. Four of these were asymptomatic but had repeat tick bites and were concerned about reinfection. Repeat serology demonstrated the presence of Borrelia antibodies, but semi-quantitative analysis of immunblot patterns did not support either continuing active infection or re-infection. No further treatment was given.
Three patients with confirmed Lyme disease reported non-specific symptoms of fatigue or myalgia at late consultation. Repeat serology in two supported a reduction in antibody concentration, interpreted as no evidence of active infection. No further treatment was given. A single patient showed strong immunoblot response and received treatment with a further 4 weeks of oral doxycycline.
In this group of patients chronic Lyme disease was not recognized. A few patients in this series presented relatively late with neuroborreliosis, having had primary Lyme missed or misdiagnosed. At least two of these patients had possible diagnoses of chronic degenerative neurological conditions made before the diagnoses of Lyme disease was made. The patient with myelitis (Fig. 5) had been told he had a probable inoperative spinal tumour and had started to arrange his own funeral! All of these patients had strongly positive Borrelia serology. They all responded favourably to antibiotic treatment without recurrence and recovery occurred without chronic sequelae. There were no cases of Borrelia lymphocytoma or acrodermatitis chronica atrophicans in this series.
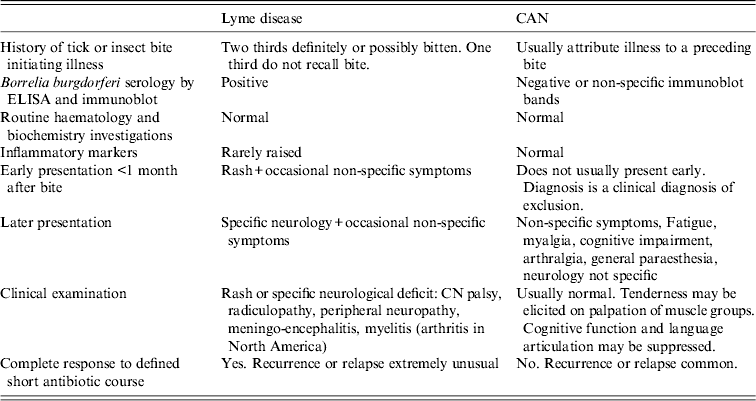
Patients with chronic symptoms such as myalgia, fatigue, cognitive impairment, muscle fasciculation following a history of tick bite who had negative or non-specific Borrelia serology and who have a diagnosis of ‘chronic Lyme disease’ have not been included in the case series because they do not fit the case definition of Lyme disease. Table 2 lists the clinical differences between Lyme disease and the proposed chronic arthropod-borne neuropathy (CAN).
Table 2. Case definitions for Lyme disease and chronic arthropod-borne neuropathy (CAN)
DISCUSSION
Main findings
This is the largest series of serologically confirmed Lyme disease cases reported in the UK. The findings mirror and confirm other reports of this infection from the UK [Reference O'Connell6, Reference Due16, Reference Cottle17] showing that Lyme disease is common in Britain, has fairly characteristic clinical presentations (skin or focal neurology with or without generalized non-specific symptoms), is readily diagnosed both clinically and by conventional serology, is effectively treated with a few weeks of antibiotics and rarely recurs or results in chronic symptoms. Confirmation of a clinical suspicion of the diagnosis is made serologically. Although serology may be negative in the early stages of disease [Reference Stanek5, Reference O'Connell6, Reference Wormser8, Reference Duncan, Carle and Seaton10, Reference Wilske11], clinical diagnosis on the basis of an expanding erythema migrans rash following a tick bite warrants early empirical treatment. A serological response may also be aborted by very early treatment of the primary lesion. Serology, once positive, tends to remain positive but does not necessarily indicate active infection. Chronic manifestations of Lyme disease are invariably associated with positive serology.
As elsewhere in northern Europe, Borrelia spirochaetes are transmitted by the hard-bodied tick, Ixodes ricinus, commonly known as deer or sheep ticks [2, Reference Stanek5, Reference O'Connell6]. These are commmon in the countryside in Hampshire. Habitats suitable for acquiring B. burgdorferi infection occur in temperate regions of the northern hemisphere, usually in forested woodland or heathland areas which support the life-cycles of ticks and the small mammals and birds that are the reservoir hosts for B. burgdorferi. This is exactly the sort of environment in the farmland, river valleys and chalk downland around Winchester. With the development of rural areas, and with the rise in popularity of countryside and athletic pursuits by more people, there has been increasing opportunity for human exposure to this zoonosis. This may partly explain the rising incidence.
The true incidence of Lyme disease in the UK is unknown, often being treated on clinical suspicion in the early stages without confirmatory serological testing. Some cases are self-limiting and will recover without treatment or sequelae. PHE data has shown the number of cases in England and Wales to have risen slowly to around 900 serologically confirmed cases a year as awareness increases [1].
The numbers of diagnosed cases in Winchester in this series has increased steadily each year from four cases in 1992 to a peak of 46 in 2009, at which point the incidence appear to have plateaued (Fig. 1). We believe this is a genuine increase, rather than heightened awareness and improved diagnosis. In 1992 one of us (M.S.D.) had a specialist interest in Lyme disease and actively sought cases in primary-care practices in the area. This was aided by discussions, educational sessions, surveillance feedback and public leaflets increased local awareness among professionals and the public. We therefore believe that this series has captured virtually all of the cases of Lyme disease by both clinical and laboratory surveillance in this area during the study period. The increase in incidence may be explained by closer contact between the human population and animal vectors. Housing development has encroached on rural and forested areas. It is not known if the small mammal density has changed, but reared game bird (pheasant, red-legged partridge) and deer populations (roe, fallow, muntjac) have markedly increased in recent decades. Media sources in March 2013 reported the highest density of deer in the UK since the last Ice Age [Reference Connor19]. It may be the closer association of human and animal hosts that is the cause of the increased incidence.
The mean annual rate of incidence of 9·68/100 000 population (range 1·6–18·4) (Fig. 1) in this series (Fig. 1) greatly exceeds the rate reported nationally by PHE [1]. This merely reflects the ideal environmental conditions for borreliosis in this part of Hampshire. There is some year to year oscillation of infection rates. This may be due to varying annual climatic conditions, ticks preferring warmer, damper conditions [Reference Dobson20]. It is likely that in those years ticks are more prevalent and more humans get bitten. It is not known whether the proportion of ticks carrying Borrelia spp. varies. Rates of Lyme disease in some European countries are much higher with an estimated incidence of 206/100 000 population in Slovenia (based on laboratory reports) and 135/100 000 population in Austria (based on physician surveys) [Reference Smith and Takkinen21].
Several pathogenic genospecies of B. burgdorferi have been identified in Europe, and there is evidence for some variation in the types of clinical presentation caused by these different genospecies [2, Reference Mygland4, Reference Stanek5]. B. garinii, the most common subspecies is associated with neurological manifestations and B. afzellii with dermatological manifestations. Other genospecies may be present too and it is possible that some of these may be responsible for chronic seronegative disease [Reference Due16]. Further contemporary research is required to establish the current distribution of British genospecies in ticks, vectors and in human infection and this may be possible by collaboration between a National NHS Lyme clinic, diagnostic services at the Rare and Imported Pathogen Department of PHE and patients’ advocacy groups such as Lyme Disease Action.
The disease classically follows a course associated with the earliest manifestations often including a typical rash (erythema migrans) usually where the organism is inoculated at the site of the tick bite, although haematogenous spread has been recognized. Almost three quarters of the patients in this series described a rash. Our findings suggest there is an interval between first symptom and clinical diagnosis of 14 days. There is no previous comparable published data from the UK. Our data are consistent with previous observations in European Lyme disease, where median incubation period from tick bite to erythema migrans has been reported as 17 days [Reference Stanek5]. The rash is probably associated with an inflammatory response at the skin site where the organisms are multiplying. Serology at this stage of presentation may be negative, but seroconversion follows shortly afterwards.
Flu-like and other systemic symptoms may follow as the rash evolves. This was a common finding in this series. When diagnosis occurs at this stage and treatment is initiated, these symptoms resolve and there is no further progression of infection. The most common secondary manifestation was neuroborreliosis, occurring weeks to months after the tick bite, sometimes with but often without a history of a rash. In Europe and in this series a predilection for neuronal tissue was high. It is likely this reflects the particular pathogenesis of the European genospecies. In the patients in this series resolution of neurological symptoms was swift after diagnosis and appropriate treatment without chronic sequelae.
Some patients present later with neurological symptoms and may not describe or recall an acute phase of illness [Reference Stanek5]. Although some clinical features may be non-specific, the main clinical presentations of European neuroborreliosis are well defined, and diagnosis is based on these with confirmatory serology. The varied nature of the clinical manifestations present a diagnostic challenge which is why concise case definitions with positive serology are essential. It is precisely for this reason that Lyme disease can not be a purely clinical diagnosis as suggested by Burrascano [Reference Burrascano22]. Conversely, as the presentation of Lyme disease is so diverse, laboratory testing by conventional methods, ELISA and immunoblot, is essential to confirm or exclude the diagnosis. Patients with Lyme disease who present late in the UK tend to have quite specific neurological signs and have strongly positive serology. Patients without specific neurological signs and chronic non-specific symptoms and negative serology are therefore unlikely to have Lyme disease, which is why we propose the new term CAN. The condition CAN needs further investigation and research, but it is confusing to call it ‘chronic Lyme disease’.
Cases of Lyme disease present throughout the year. Time of tick bite, time of first clinical symptom and time of diagnosis follow a seasonal variation, with a peak in the early summer months, coinciding with major ixodid tick feeding activity [Reference Dobson20]. The most common reported months for tick bites in this series were May–July. The main months for reporting of first symptoms and of serological diagnosis were June–August. The US Centers for Disease Control and Prevention (CDC) data also demonstrates a similar bimodal age distribution with peaks at ages 0–10 years and 40–70 years [23]. The reason for this observation remains unclear, but may relate to health-seeking behaviour, or it may just represent the ages at which people spend greatest time in the countryside and therefore at greatest risk of exposure.
Half of the patients described a preceding tick bite and 71% had a rash at presentation. The dermatological manifestations of Lyme disease predominantly affected the lower limbs. Arthralgia was present in 8% of patients, but without true clinical arthritis, unlike in comparable US studies and consistent with our limited knowledge of the effect of genospecies on manifestations of disseminated disease. B. burgdorferi sensu stricto, the genospecies most strongly associated with Lyme arthritis is rare in the UK, but the most common pathogenic species in the USA [Reference Wormser8]. This difference may reflect differences in the antigenic structure of USA and European strains [Reference Strle24, Reference Baranton25]. Cardiac involvement (myocarditis, conduction abnormalities) was uncommon and in our experience, usually transient.
Neuroborreliosis, as characterized by clear clinical neurological signs, was present in 16% at presentation and the organism demonstrated a predilection for involvement of the facial nerve, although other cranial nerves, spinal ganglia and even central nervous system (CNS) parenchyma were also involved. At least three patients with more severe neuroborreliosis [meningoencephalitis (n = 1), radiculopathy (n = 1), myelitis (n = 1); Fig. 4] presented later having not been diagnosed when they presented with a primary rash. Presentation was at 2–6 months after the rash. All recovered on antibiotic treatment. There have been no long term sequelae. This is consistent with a report of long-term follow up of a large series of serologically confirmed borrelial meningoradiculitis and encephalomyelitis which despite being untreated with antibiotics resolved spontaneously without long-term or progressive complications comparable to neurosyphilis [Reference Kruger26]. Nevertheless some patients can experience delay in diagnosis, depending on the attending physician's experience of the disease and index of suspicion for it [Reference Hobson and Weatherall27]. Of the 20% of patients in our series who described ‘flu-like’ or non-specific symptoms and were serologically positive, these symptoms resolved rapidly on antibiotic treatment. This is not the case in a US series where some patients with confirmed serology, as well as those without, had prolonged non-specific symptoms [Reference Klempner28]. Neither group demonstrated clinical improvement on prolonged antibiotics.
As there were no cases of Borrelia lymphocytoma or acrodermatitis chronica atrophicans in this series, one must postulate that in this UK hotspot of Lyme disease, these conditions may not exist, they may be rare, or all symptomatic Lyme disease is being diagnosed and treated at an early stage, thus preventing progression to this chronic pathology.
All patients in this series responded to courses of antibiotics recommended in current guidelines and which were given for 14–28 days in most cases. Only one case of complex neuroborreliosis received antibiotics for 12 weeks [Reference Dryden29]. This was a clinical decision based on the severity of the MRI findings and resulted in complete resolution of symptoms and the radiological abnormality (Figs 4, 5). It would appear that prolonged antibiotic courses do not improve chronic symptoms [Reference Klempner28]. It is also debatable whether intravenous antibiotics improve outcome compared to oral antibiotics in neuroborreliosis [Reference Kruger26]. Research is warranted into the duration of intravenous antibiotics before oral treatment can be safely initiated [Reference White, Seaton and Evans18]. It has been suggested that the duration of antibiotics for Lyme disease in existing guidance could be shortened [Reference Wormser and O'Connell30].
Chronic arthropod-borne neuropathy (CAN) instead of ‘chronic Lyme disease’
There is another disease which is becoming increasingly common which is often labelled ‘chronic Lyme disease’. The number of such referrals is rising as patients are increasingly aware of the condition and have greater access to information through the internet and other resources. These patients are not included in the numerical data in this series, but are worthy of further discussion.
This disease remains an enigma. If it is an infection following an arthropod bite, it is likely to be a different infection from the B. burgdorferi infection identified in the patients in this series. If it is not an infection the aetiological possibilities are varied and could be toxin-related, immunological derangement, autoimmune disease or psychological illness. The chronic syndrome presents with non-specific, non-focal neurology, has negative or equivocal Borrelia serology, does not respond well to antibiotics and is recurrent, relapsing and persistent (Table 2).
Many patients are self-diagnosed by entering symptoms on the internet. Many websites and patients’ advocacy groups now support this diagnosis and conflict has arisen between patients’ groups and conventional medical practitioners who cite lack of scientific evidence for chronic, seronegative Lyme disease [Reference Auwaerter31]. In addition a number of alternative practitioners and non-validated scientific diagnostic tests support the patients with ‘chronic Lyme’ and this increases the general confusion around this illness.
The patients referred with ‘chronic Lyme disease’ all have non-specific signs of various combinations of fatigue, headache, myalgia, paresthesia, cognitive suppression and depression and are Borrelia spp. seronegative or ‘equivocal’ by ELISA and immunoblot testing. Routine haematology, biochemistry, endocrinology, autoimmune investigations and inflammatory markers are generally normal in these patients, as indeed they are in seropositive Lyme disease. Some of these patients have received courses of antibiotics and had not shown a clinical response; others describe an improvement in their symptoms following antibiotic therapy. A few patients request intravenous or prolonged antibiotic treatment on the basis of non-evidence-based recommendations [23]. A significant proportion of these patients have invested in alternative blood tests offered by private clinics and performed using techniques that are not scientifically validated and unrepeatable, at least by UK standards [Reference Auwaerter31]. These tests include non-validated immunoblot and PCR methods, and indirect tests such as lymphocyte transformation tests and measurement of CD57 lymphocyte subsets as markers of borrelial infection. Such tests are commonly reported as positive not only for B. burgdorferi but also for co-existent pathogens such as Anaplasma sp., or Ehrlicia sp. and Babesia sp. Such results reinforce the patient's own belief that active microbial infection is the direct cause of their symptoms. In addition certain organizations and their websites [14] support borrelial infection as a contributor to a wide range of chronic neurological conditions such as multiple sclerosis, atrophic lateral sclerosis, Parkinson's disease and even vascular dementia. Patients with genuine, distressing symptoms need additional support, further investigation and research [Reference Pearson and Huyshe-Shires32], but they do not deserve to be duped by a parallel universe of quasi-science [Reference Auwaerter31].
It is difficult therefore to support a syndrome of chronic Lyme disease as described on numerous websites. This series has shown that patients with clear objective neurological signs who have positive Borrelia serology recover swiftly on antibiotic treatment. Patients with non-specific signs that might involve the CNS with positive Borrelia serology also recover swiftly on antibiotic treatment. The Kruger study demonstrates that untreated CNS borreliosis is a relatively benign disease with spontaneous recovery even though seropositivity in the CSF may be present years later [Reference Baranton25]. Patients with chronic non-specific neurological symptoms that are Borrelia seronegative tend not to recover with or without antibiotic treatment [Reference Klempner28], suggesting that the pathogenesis of the illness is unrelated to B. burgdorferi.
This syndrome should therefore have a different name while its aetiology is being investigated. We suggest chronic arthropod-borne neuropathy (CAN), rather than chronic Lyme disease. CAN encompasses the perceived cause, a tick or insect bite, the chronic nature of the illness and the non-specific neurological symptoms. The following could serve as a case definition: previously well patient, history of a tick/insect bite, plus a subsequent illness involving a combination of symptoms including: fatigue, myalgia, paresthesia, headache, cognitive impairment, normal clinical examination, negative B. burgdorferi serology by validated ELISA and immunoblot, normal routine haematology, biochemistry, inflammatory markers, endocrinology, and autoimmune profile.
Patients with CAN require further investigation into the aetiology and pathogenesis of their illness. This is supported by the British patient advocacy group, Lyme Disease Action [12]. The key research requirements are:
-
(1) To follow the serological response by immunoblot of primary Lyme disease to establish the changes in antibody development to different Borrelia antigens throughout the course of the infection and to compare and validate novel diagnostic methods.
-
(2) Molecular and nucleic acid amplification of skin biopsy and blood in Lyme and CAN for other strains and species of Borrelia and for other pathogens.
-
(3) Molecular and nucleic acid amplification of ticks from around the UK for other strains and species of Borrelia and for other pathogens.
-
(4) Establish a clear distribution map for Lyme disease risk in the UK based on the results of item (3).
-
(5) Placebo-controlled trials of antibiotics or immunomodulators in CAN.
-
(6) Further research into the causes of chronic symptoms in patients with CAN.
CONCLUSIONS
Lyme disease is common in Hampshire and is increasing. It typically presents with skin or clinically evident neurological signs, but as these presentations may be diverse, clinical suspicion is important for the acute physician. Serological testing confirms the diagnosis, but treatment should not be delayed when a typical primary rash follows a history of a tick bite. Treatment with appropriate antibiotics is effective. Recurrence or chronic Lyme disease is very unusual in our experience in treated seropositive borreliosis. Chronic symptoms with negative or equivocal serology are unlikely to be active borrelial infection, and it would be helpful to patients and doctors alike to separate this condition from Lyme disease. A separate name, chronic arthropod-borne neuropathy (CAN), is proposed. This condition requires much further research.
ACKNOWLEDGEMENTS
We thank colleagues in microbiology, the wards and in general practice in the catchment area of the Royal Hampshire County Hospital, Winchester, UK for their collaboration in the diagnosis and management of all these patients. We also acknowledge the invaluable diagnostic assistance of Dr Sue O'Connell and her laboratory colleagues in the HPA, now PHE Lyme reference laboratory. In addition we value the positive discussions we have recently had with Lyme Disease Action over the complex issues surrounding chronic symptoms following tick bites. We hope that these will lead to future collaboration and research. This study had no specific funding.
DECLARATION OF INTEREST
None.










