Introduction
Over the past 15 years, the Americas have seen an unprecedented number of reported dengue cases with an alarmingly increasing trend [Reference San Martin1–Reference Bhatt5]. Latin America and the Caribbean have reported the highest incidence of dengue worldwide with over two-thirds of all cases reported from this region during the 2000–2006 [Reference San Martin1, Reference Shepard2]. In 2010 and in 2013, the English-speaking Caribbean was one of the worst affected regions in the Americas [Reference Cafferata3, 6, Reference Chadee, Mahabir and Sutherland7]. Dengue is reported to be hyper-endemic in most of the English-speaking Caribbean, including Barbados, with an epidemic occurring every 4–5 years during the period 1997 through 2009 [Reference Chadee, Mahabir and Sutherland7–Reference Campione-Piccardo9]. All four serotypes of dengue have been circulating in the Caribbean and since 2001, all of the serotypes have been concurrently isolated in some of the countries, including Barbados [Reference Campione-Piccardo9, Reference Gittens-St Hilaire and Clarke-Greenidge10]. The long-term patterns of dengue incidence have been studied at numerous endemic sites in south-east Asia [Reference Campbell11, Reference Thai12]. There are some meta-analyses on the long-term incidence pattern from the Americas [Reference Kumar8, Reference Stoddard13–Reference Teixeira17]. Results from these studies highlight intra-annual (seasonal) and inter-annual (across multiple years) signatures in the transmission intensity [Reference Campbell11–Reference Stewart-Ibarra and Lowe16], as well as shifts in the age of people with clinically apparent illness [Reference Stewart-Ibarra and Lowe16, Reference Teixeira17]. Conclusions from these studies are mixed, although, in aggregate, they highlight that dengue occurs across a diverse array of conditions and that the key drivers of transmission vary across those different conditions [Reference Campbell11–Reference Teixeira17]. Continued and detailed documentation of these temporal dengue patterns in different endemic populations is useful for improving our understanding of the dynamics of dengue epidemics as well as DENV transmission and for testing the link of key variables like rainfall to components of the virus transmission cycle.
Barbados, one of the English-speaking Caribbean countries, is a commonwealth, independent nation and has a total population of 285 000 (2016) including 54 599 (22%) under 15 children and a population density of 662.8/sq km. The majority (90%) of the population are of African descent [18]. The Government of Barbados provides free health care to its citizens through polyclinics, which are the primary care centers and a single tertiary care hospital. People also use private healthcare facilities by paying out of pocket. Barbados has an Active Dengue Surveillance System (ADSS) in place since 2000. Under this system, all the cases of suspected dengue are tested for confirmation and the testing is provided free of charge to all patients attending both public and private healthcare facilities. There is a central dengue testing laboratory. Patients with suspected dengue are usually managed at the discretion of the attending physician along the WHO guidelines and blood samples for dengue confirmation are routinely collected from all suspected cases at the time of presentation or on a follow up visit around day 5 of illness, in case patients present during day 1 or 2 of their illness. Taking advantage of the unique health care system in this closed population, we used the ADSS to study the long-term epidemiologic and the seasonal dynamics of dengue in Barbados.
Material and methods
Study design and patient selection
This is a prospective population-based study. The study period extends from 2006 to 2015. Cases of suspected Dengue were prospectively identified from the register at the central dengue laboratory. All suspected cases of dengue in adults and children from both, the private care settings and the public settings of the polyclinics and the hospital were included in this study. Cases of dengue among visitors to this island were excluded from this study. A preplanned analysis of relevant epidemiological data on all the suspected and confirmed cases of dengue and the local meteorological data were used to describe the dynamics of dengue transmission in this population. Local data were also compared with the available regional data obtained from the Pan American Health Organization (PAHO) for similarity and differences, if any, in the transmission dynamics between Barbados and other Caribbean countries which share similar climatic conditions.
Patient data
Demographic data such as the age, gender, date of onset of the illness and timing of the blood collection for dengue testing were prospectively abstracted from their laboratory requisition form. Any missing information with respect to the demography, date of onset or the timing of blood collection for any of the suspected cases were obtained from the patients case notes at the clinics where the patient was seen.
Laboratory confirmation
As per established protocol at the dengue laboratory, blood samples collected during the first 4 days of the illness are tested for the NS1 antigen along with IgM antibodies and blood samples collected after day 5 of illness are tested for the Dengue IgM antibodies. NS1 antigen testing is done using Platelia™ Dengue NS1 Ag-ELISA (Biorad Laboratories, Marnes-La-Coquette, France). Dengue IgM antibody-capture ELISAs (Focus Diagnostics, Cypress, CA, 90630 USA) were used for the IgM detection. The dengue test results for all the cases of suspected dengue were actively followed up. In those cases where the blood sample was negative and the blood sample was taken during the first 4 days of the illness then the patients’ attending physician was advised to request a second blood sample from the patient to be taken during day 5–15 of the illness for retesting.
Local meteorological data
Data on the annual rainfall, humidity and temperature for Barbados over the study period were obtained from the Meteorological department, Government of Barbados. Data on the local serotypes circulation, both the current and the past, were collected from our central dengue laboratory in Barbados.
Regional data
Additional data on the reported number and incidence of dengue for the Caribbean were collected from the PAHO website [Reference Alera19]. These data were used to compare the local dengue transmission pattern with those of the other Caribbean and Latin American countries with similar climatic conditions.
Case definition and outcome measure
As per the guidelines from the Ministry of Health, dengue is suspected in any person presenting with fever of duration more than 24 h with one or more signs and symptoms of dengue such as aches and pains, vomiting or nausea, rash, bleeding, abdominal pain, lethargy or hepatomegaly and where other causes of the fever are not obvious at the time of first presentation. A confirmed case of dengue was defined as one that had a positive IgM titer and/or positive NS1 (non-structural protein 1) antigen. The overall mean annual incidence rate was calculated by dividing the total number of cases over the study period by the population (based on 2010 census) and expressed as a percentage. The annual incidence rate was calculated by dividing the number of cases for the year by the population (based on 2010 census) and expressed as a percentage. The number and the incidence rate of both suspected and the confirmed dengue detected during this prospective surveillance study were compared with those reported from other Caribbean countries to the PAHO [Reference Alera19]. The years where the number of cases of dengue showed a peak were designated as epidemic years.
Data management and statistical analysis
Data were stored in a specially designed Microsoft® Access database and was analysed using SPSS® statistical software package version 11. Proportion and 95% confidence interval (CI) were calculated and results were corrected for continuity. Associations between categorical variables were assessed for statistical significance by χ 2 test. A P value of ⩽0.05 was considered statistically significant. All P values were two-tailed.
Ethics approval
Necessary ethical approval for this study was obtained from the Institutional Review Board on Ethics in Research on human subjects at the University of the West Indies.
Results
Over the 10-year study period there were 13 991 cases of febrile illnesses where dengue was suspected and a sample was sent for dengue confirmation. Over the same period, 4344 (31.10% those tested) cases were confirmed as dengue. Diagnosis of dengue was confirmed based on a positive IgM serology in 3840 (88.4%) cases, based on a positive NS1 antigen in 384 (8.8%) cases and based on both NS1 and IgM positive results in 120 (2.8%) cases. All 13 991 cases had at least one sample tested for IgM antibody and 3960 (28.3%) cases were positive. NS1 antigen test was done in 1448 cases and 504 (34.8%) were positive.
The overall mean annual incidence rate of suspected and confirmed dengue over the study period was 0.49% (Range 0.15%–0.99%) and 0.16% (Range 0.05%−0.48%), respectively, compared with the reported pooled mean annual incidence of confirmed cases for the Caribbean at 0.058% (P < 0.001), over the same period. Table 1 shows a comparison of the number and the incidence rate of the suspected and the confirmed dengue reported in this study with those reported from other countries in the Caribbean region to the PAHO. In Barbados, the number of both the suspected and the confirmed dengue peaked during the years 2007, 2010 and 2013 and were designated as the epidemic years. The reported number and the incidence of suspected and confirmed dengue reported to the PAHO from the other countries in the Caribbean also peaked in 2007, 2010 and 2013. However, the incidence rate of dengue in Barbados during these epidemic years was significantly higher than those of other Caribbean countries (P < 0.001). In Barbados, DENV3 was the only serotype in circulation during 2006–2008. The DENV2 appeared in 2009 when both DENV3 and DENV2 were in circulation. In 2010, DENV 1 was reintroduced along with the introduction of DENV4 and all four serotypes were simultaneously in circulation. Since 2011 up until the end of 2013, DENV1, DENV2, DENV4 were simultaneously in circulation. Since 2014 only DENV1 has been in circulation.
Table 1. Dengue incidence and serotype circulation in Barbados compared with those reported from other Caribbean countries to Pan American Health Organization (PAHO) during 2006–2015
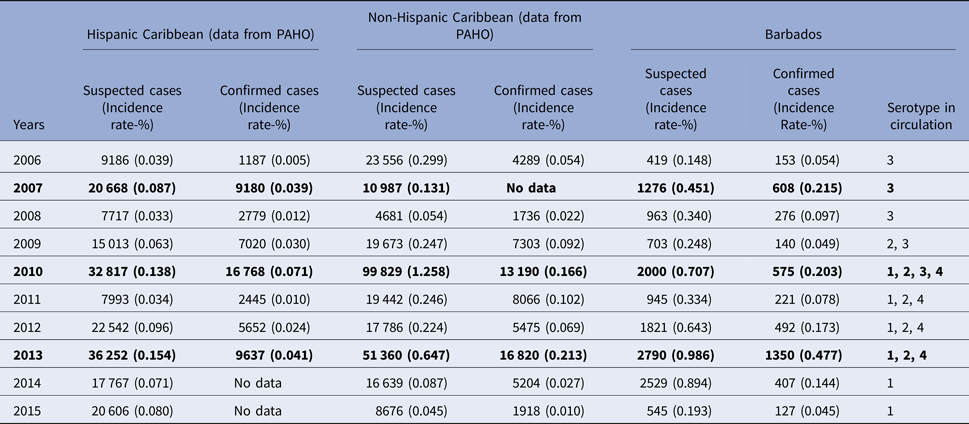
Note: numbers in bold are those for the epidemic years for Barbados.
The annual number of suspected and confirmed cases along with the annual rainfall pattern in Barbados is shown in Figure 1. The annual total rainfall peaked during the years 2008, 2010 and 2013 and coincided with the peak number of cases in 2010 and 2013. The total annual rainfall troughed in 2009, 2012 and 2015 and coincided with the trough number of both the suspected and the confirmed cases during 2009 and 2015. However, no significant correlation was seen between the total annual rainfall and the total annual number of suspected (P = 0.2049) or confirmed cases (P = 0.4045). Figure 2 shows the mean monthly climatic data and the mean monthly number of cases during the 10-year study period. When the mean monthly climatic data were analysed with a monthly number of cases, there was a significant correlation between the mean monthly number of confirmed cases and the mean monthly rainfall (P = 0.0026) and with the mean monthly relative humidity (P = 0.0142). No significant correlation was found between the monthly cumulative number of confirmed cases and the minimum or maximum temperature.

Fig. 1. Total annual rainfall and the number of dengue cases in Barbados, 2006–2015.
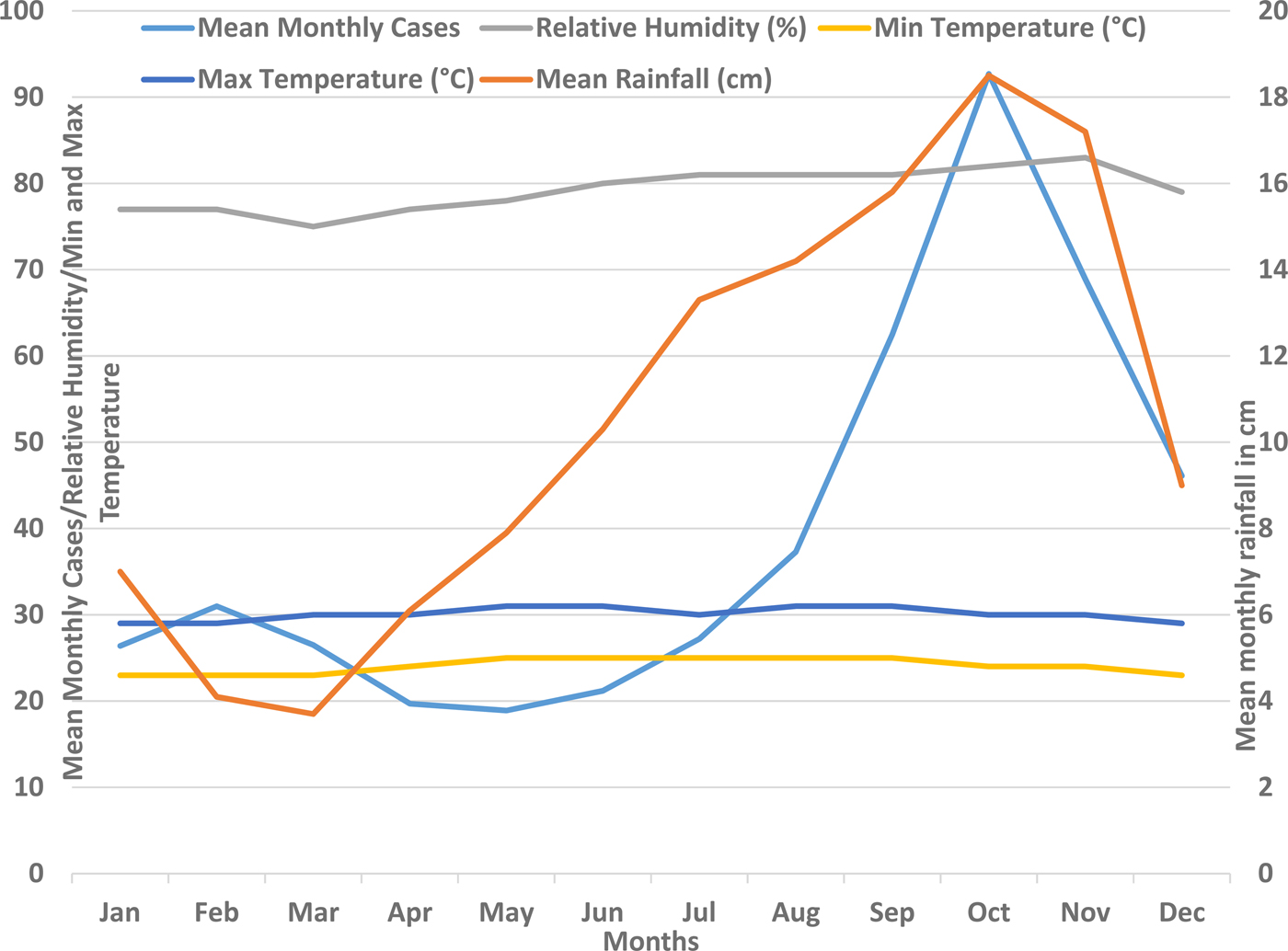
Fig. 2. Mean monthly rainfall and mean monthly number of confirmed dengue cases in Barbados, 2006–2015.
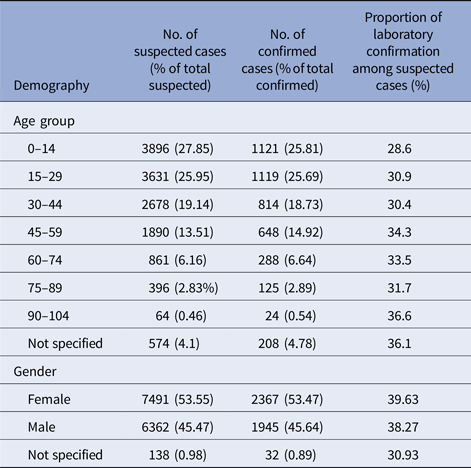
The overall age and sex distribution of the suspected and confirmed cases are shown in Table 2. The median age of the patients with both, suspected and confirmed dengue was 25 years with the highest proportion of cases seen in the age group 0–15 years. The difference in the proportion of suspected cases (P < 0.0001) and confirmed cases (P < 0.0001) in each age group category was statistically significant. Females had a significantly higher proportion of both the suspected (P < 0.0001) and confirmed cases (P < 0.0001) compared with males. The difference in the proportion of suspected cases that was confirmed dengue in the various age groups was statistically not significant (P = 0.062). The proportion of suspected cases that were confirmed dengue during the epidemic years was at 41.53%, whereas the corresponding figure during the non-epidemic years was at 24.94% and this difference was statistically significant (two-tailed P = 0.0165).
Table 2. Demographic characterisation of the dengue epidemics in Barbados, 2006–2015
The mean annual incidence rate of suspected cases among children (<15 years) and the adults were 0.75% and 0.43% respectively, and this difference was statistically significant (P = 0.0026). The annual incidence rates of suspected and confirmed cases among children and adults (overall), among children and among adult were analysed and they all showed an upward trend during the 10-year study period. The annual incidence rate of suspected cases of dengue is shown in Figure 3. During the study period, there was an increasing upward trend noted in the annual incidence rate of suspected dengue among children and this upward trend was statistically significant (R 2 = 0.2424). An increasingly upward trend in the annual incidence rate of suspected dengue among adults was also noted, however, this was statistically not significant (R 2 = 0.199). During the peak (epidemic) years 2007, 2010 and 2013 there was a consistent increase in the overall (children and adults) annual incidence rate of suspected dengue and this increase was highly significant (P = 0.0002). During the trough years 2006, 2009, 2011 and 2015 change in the overall annual incidence rate of suspected dengue was inconsistent and varied within a narrow range (0.15% and 0.33%). The difference in the annual incidence rates of suspected cases during the trough years was statistically not significant (P = 0.0621). The mean annual incidence rate of confirmed dengue among children and adults was 0.23% and 0.13%, respectively, and this difference was statistically significant (P = 0.0022). The pattern in the annual incidence rate of confirmed dengue in children and in adults during the study period and during the epidemic and non-epidemic years mirrored the pattern seen for the suspected cases (Fig. 4).

Fig. 3. Incidence rate of suspected dengue in children and adults, Barbados, 2006–2015.

Fig. 4. Incidence rate of confirmed dengue in children and adults, Barbados, 2006–2015.
Discussions
Results from this longitudinal study reveal the dynamics of dengue epidemiology in Barbados. There are several long-term studies, mostly from south-east Asia on dengue transmission that have sought to define the dynamics of dengue epidemiology [Reference Thai12, Reference Alera19–Reference Branco21]. However, more studies are required from the Caribbean to quantify the disease burden in different populations, explore the impact of DENV serotype-specific transmission on host-responses and dengue severity and measure the economic impact of dengue in this population. A long-term dengue transmission study is especially required from the English-speaking Caribbean, where there is a high intensity of transmission and paucity of such studies [Reference Kumar8, Reference Campione-Piccardo9, Reference Kumar and Nielsen22]. Findings from this long-term prospective study provide the epidemiologic data that is important for describing the dynamics of dengue epidemiology and for planning control measures [Reference Murray, Quam and Wilder-Smith4, Reference Endy, Yoon and Mammen23, Reference Ramadona24]. The findings from this study are especially important in the context of the fact that Latin America and the Caribbean have been projected to be the region of the highest dengue transmission in the world with increasing frequency and intensity of dengue epidemics over the past decade based on data that is limited in terms of quality, completeness and coverage [Reference San Martin1, Reference Cafferata3, 6, 25].
Dengue in Barbados occurs throughout the year with cases occurring each month in every year throughout the study period. Epidemics of dengue were seen in 2007, 2010 and 2013, all these 3 years also witnessed a pan-Caribbean epidemic (Table 1) and the years 2010 and 2013 witnessed a Pan-American epidemic [6]. The inter-epidemic period of 3 years seen in this country is shorter than the 3–5 years seen in our previous report over the period 2000–2009 [Reference Kumar8]. Similar observations of shorter inter-epidemic durations have been made in some other recent studies from the Americas [Reference Teixeira26, Reference Villar27]. The mean annual incidence of suspected dengue over the study period in this population was 0.49% (Range 0.14%−0.99%). This was significantly higher than the reported pooled mean annual incidence of suspected cases for the Americas (Latin America and the Caribbean countries) at 0.072%, over the same period [Reference Cafferata3]. It is noteworthy that although the Hispanic and Non-Hispanic (English-French-Dutch-speaking) Caribbean have geographic proximity and share similar climatic conditions [Reference Méndez-Lázaro14, Reference Gharbi28], Barbados in particular and rest of the non-Hispanic Caribbean, in general, have consistently recorded a significantly higher incidence rate of dengue during the epidemic years as compared with the Hispanic Caribbean [6]. More movement of people among the different English-speaking Caribbean countries with greater potential for different serotypes introduction and reintroduction could be a factor in the higher incidence of dengue in these countries [Reference Vaughn29]. This is further supported by the fact that the circulating serotypes in the neighboring English-speaking Caribbean are similar to those circulations in Barbados [Reference Campione-Piccardo9]. Higher population density in Barbados compared with the other Caribbean countries may also facilitate greater transmission of dengue in this population. Lastly, this higher incidence rate of dengue in Barbados, as seen in this study, compared with the number of cases reported to the PAHO from Barbados and other Caribbean countries may partly be due to the underreporting. Most of the Caribbean and Latin American countries use national passive surveillance for reporting dengue to the PAHO and under-reporting is a well-recognised problem in this system. Several studies from the Latin Americas have shown that the number of dengue reported to the PAHO are several folds lower than the actual number of cases [Reference Standish30, Reference Sarti31].
The annual incidence rate of dengue in Barbados, at least for some of the years during the study, was also higher than that reported from some other hyper-endemic countries in the Americas [Reference Teixeira26, Reference Villar27]. For the year 2010, Brazil reported an annual incidence rate of 0.5% and Columbia reported an incidence rate of 0.6% as compared with our own rate of 0.71% [Reference Teixeira26, Reference Villar27]. A higher number of cases were seen in Barbados, as compared with both, the Americas as a whole and the English-speaking Caribbean, during all of the epidemic years [6]. Whether the higher transmission of dengue, in general, over the study period in this country, is due to the variation in the rainfall pattern in the region, the intensity of vector control measures or due to the higher population density of Barbados, needs further study.
During 2014 and 2015, the pattern of dengue occurrence in Barbados and other English/Dutch/French-speaking Caribbean have reported a lower number and incidence rate of dengue compared with the Americas as a whole [6]. The intensity of DENV transmission through the years – with the circulation of several serotypes, as shown in this study – may have led to the decrease in the number of cases. This decline may also be due to better fogging and vector control measures after the massive 2013 epidemics. These dynamics of dengue transmission was further complicated by the introduction and subsequent epidemics of chikungunya in this population during the 2014 and 2015 and its possible effects on dengue transmission given that they both share the same vector [Reference Kumar, Best and Benskin32].
A closer look at the data for the Caribbean region reveals progressively higher peaks and troughs in the number and incidence of dengue over the study years. However, in Barbados and other English-speaking Caribbean, although the peaks have had a progressive rise, the troughs have not shown a similar rise, during the study period (Table 1). This lack of carrying forward of high-intensity viral transmission of the epidemic into the following years may be due to the intense fogging and other mosquito control measures triggered during the epidemic and continued for several months thereafter. This can be described as a ‘need based mosquito control program’.
Although a higher number of dengue cases were seen during the years with more rainfall, compared with the years with less rainfall (Fig. 1), no significant correlation between the mean annual rainfall and number of cases was seen over the study period. Analysis of monthly cases and weather patterns showed that dengue cases consistently peaked during the rainy months of the year during all of the study years. There was a significant correlation between the mean monthly number of cases and the mean monthly rainfall as well as the relative humidity (Fig. 2). These findings support the facts that the rainfall distribution and density, and its interplay with other weather conditions such as temperature and humidity are important for vector density and dengue transmission than simply the amount of rainfall [Reference Stoddard13, Reference Méndez-Lázaro14]. The dengue occurrence and weather patterns suggest a complex relationship rather than a simple one, as also reported in some other more elaborate studies on dengue and climatic factors [Reference Stoddard13, Reference Méndez-Lázaro14]. It is likely that the distribution pattern of the rainfall and its interplay with temperature and other weather variables plays an important role in the vector breeding and dengue transmission. In recent studies from Puerto Rico (Spanish-speaking Caribbean) and Guadeloupe from this region, it was shown that the occurence of dengue cases were significantly associated with multiple weather variables [Reference Méndez-Lázaro14, Reference Gharbi28].
Dengue in this population is predominantly a childhood infection with the highest proportion of cases seen in the age group 0–15 years. Another population-based longitudinal study of dengue, both in children and adults from Philippines, which is also hyper-endemic for dengue, has also reported the highest proportion of cases in the age group 0–15 years [Reference Alera19]. However, a similar age distribution pattern has been reported in a systematic literature review of retrospective studies from some other countries in the Latin America [Reference Teixeira26, Reference Villar27, Reference Do33]. The median age of the patients with confirmed dengue in this population has decreased from 27 years in a previous study of dengue during the 2000–2009 from this country, to 25 years in the present study [Reference Kumar8]. A similar shift in the age of dengue has been seen in some other long-term studies from the Americas [Reference Villar27, Reference Dante´s, Farfa´n-Ale and Sarti34]. In south-east Asian countries, where all the serotypes (DENV-1–4) are circulating, DF is typically acknowledged to be a disease of early childhood [Reference Dante´s, Farfa´n-Ale and Sarti34]. This is expected to happen when there is a long lasting transmission of the viruses in one setting; adults have been infected with all four serotypes and this trend pushes infection towards adolescents and children. When dengue is introduced into a naive population, it predominantly affects the adults [Reference Bhatia, Dash and Sunyoto35, Reference Rahman36]. Infection with any one dengue serotype confers lifelong immunity to that serotype and repeated exposure with different serotype over time results in decreasing pool of susceptible adults. Studies from south-east Asia have shown a similar shift in the affected age group pattern [Reference Alera19, Reference Olkowski37]. A previous sero-epidemiologic study in this population and another study from the region showing a high dengue sero-prevalence by early adulthood explains the age distribution of symptomatic dengue in this population [Reference Kumar and Nielsen22, Reference Morrison38, Reference Dayan39].
Another notable observation in this study was that of a significantly higher proportion of both the suspected and the confirmed cases of dengue among females which is unlike the high male to female ratios reported in studies from south-east Asia [Reference Prasith40, Reference Anker and Arima41]. A male to female ratio similar to ours has been observed in other countries from the Americas [Reference San Martin1, Reference Morrison38]. Aedes aegypti mosquitoes are mostly indoor in their habitat. Culturally, in this population, as in many others, females are more likely to be indoor thereby increasing their chance of mosquito bites and risk of dengue. However, the sex distribution observed in a surveillance study may be reflective of the demography of this country with a higher proportion of females (51.9%) in the general population [18].
The overall trend in the annual incidence rate of both suspected and the confirmed dengue in this country over the 10-year study period has been upward. The highest incidence rate of 0.48% and 1.0% for the confirmed and suspected dengue respectively was noted during the worst epidemic in 2013. Of note, the incidence rates of both the suspected and confirmed cases of dengue have progressively peaked at higher levels during the epidemic years. Despite the yearly variations and cyclical epidemics, trend analysis of the incidence of dengue in the period 2006–2015 showed an overall increase in incidence over time that was statistically significant. A similar trend has been observed in reports from some other countries in the Americas [Reference Teixeira26, Reference Villar27, Reference Bhatia, Dash and Sunyoto35]. Increasing incidence rate was more pronounced and significant among children as compared with adults where a similar increase in incidence was noted but it was statistically not significant. This finding was observed in the absence of any dramatic change in the climatic variables during the study period. As explained earlier, this disproportionate increase in incidence rate among children with shifting of the median age of dengue to younger age is expected from the long-standing transmission in this setting.
The major limitation of this study is that not all persons who have dengue symptoms always see a doctor. Persons with dengue without febrile illness or other symptoms would have been missed in this study. Also, younger children who may present with either nonspecific febrile illness or atypical features such as gastrointestinal symptoms can be diagnosed with other viral syndromes and dengue may not have been suspected. Therefore, the incidence rate of symptomatic confirmed dengue in this study may have been underestimated. Another important limitation is that inadequate data were available on other circulating viruses with clinical presentations similar to dengue especially, Chikungunya and Zika. Febrile patients may be infected by other viruses than dengue; the use of reported cases in a setting where other arboviruses circulate may over-represent the incidence rate of suspected dengue.
In conclusion, there is a high transmission rate of dengue in this country and it is higher than those reported from the Hispanic Caribbean countries. A shorter inter-epidemic period was seen in recent years. There was an inconsistent relationship between the annual rainfall and the dengue occurrence, however, a more consistent relationship was observed between mean monthly rainfall as well as mean monthly humidity percentage and dengue occurrence. These facts point to a complex relationship with weather conditions especially rainfall and humidity and dengue transmission. The most important finding from this study was that the highest incidence of dengue was seen among children. As compared with an earlier reporting period there is a shift in the dengue occurrence to the lower age group. Overall, there was an increasing trend in the incidence of dengue in this population. This increasing trend was more pronounced in the incidence rate among children as compared with the adults.
Acknowledgements
We thank Miss Nicole Clarke-Greenidge at the Dengue public health laboratory for her assistance in data collection. We also thank public health nurse responsible for dengue reporting and surveillance for her assistance with cross-checking of data. We thank Prerna Singh and Pranav Kumar Singh for checking the manuscript for English language.









