Introduction
Antimicrobial resistance is a limiting factor for the successful treatment of infectious diseases and is associated with increased patient morbidity and utilisation of healthcare resources [Reference Amadeo1, Reference Robert2]. A number of factors are associated with the emergence of resistance, one of the most important being indiscriminate or inappropriate antimicrobial use [Reference Lipsitch, Bergstrom and Levin3, Reference Heritage4]. Antibiotic resistance has affected both primary and secondary healthcare sectors. Based on epidemiologic evidence, hospitalisations pose risks for the emergence of resistance during the hospital stay and post-discharge of patients primarily due to the transmission of resistant bacterial strains [Reference Goossens5, Reference Costelloe6]. In addition to the length of hospital stay, having surgery and the presence of in-dwelling devices also impact on infection rates [Reference Struelens7]. During hospital admissions, efforts should be focused on prescribing patterns of antimicrobials and stewardship strategies to combat resistance.
Against the background of a major Clostridium difficile infection outbreak in 2008, regional funding was allocated for a specialist pharmacist in each hospital Trust to deliver on national/regional strategies for antimicrobial resistance control and stewardship. These pharmacists are members of the Antimicrobial Management Team (AMT) and are involved in setting targets, undertaking audits and devising improvement strategies when problems are identified. Their appointment ensured that all Trusts had specific, evidence-based empirical antimicrobial guidelines with the timely review. Antimicrobial stewardship ward rounds were introduced, and a programme of restriction and surveillance of use of high-risk antimicrobials was initiated.
Surveillance monitoring of prescribing patterns and quality indices as a tool to direct patient care can provide key information regarding antimicrobial outcomes, and ideally, such data need to be continuous. However, this approach is time-consuming and not feasible in practice. A point prevalence survey (PPS) for antimicrobial consumption (i.e. collecting data at certain time points) addresses this limitation by providing standardised, validated and long-standing monitoring of antimicrobial usage to inform decision-making regarding antimicrobial management [Reference Ansari8].
The present study aims to evaluate prescribing patterns of antimicrobials and quantify progress in relation to targets for quality improvement of the prescription of antimicrobials in Northern Ireland's secondary care sector using three repetitive PPS over a 6-year period. The first two were conducted within the European Surveillance of Antimicrobial Consumption (ESAC-PPS) project [Reference Zarb9], and the other was conducted for the Global Point Prevalence Survey on Antimicrobial Consumption and Resistance (Global-PPS) in 2015. This ongoing project assesses the global prevalence of antimicrobial use and resistance in hospitalised adults and children worldwide (www.global-pps.com).
Methods
The study was carried out in three major acute secondary care hospitals from distinctive geographical areas in Northern Ireland: Antrim Area Hospital (426 beds), Ulster Hospital (561 beds) and Craigavon Area Hospital (426 beds) in the Northern Health and Social Care Trust (NHSCT), South Eastern Health and Social Care Trust (SEHSCT) and Southern Health and Social Trust (SHSCT), respectively.
PPS were carried out in June 2009 (all three hospitals), June 2011 (Antrim Area Hospital and Ulster Hospital) and May 2015 (all three hospitals). Data regarding the patterns of antibiotic use were collected from each ward on a single day following the ESAC and Global-PPS audit tool guidelines, the two protocols hereafter referred to as PPS tool (www.global-pps.com) are similar and allow comparison between the different time points. Inpatients present in the surveyed ward at 8 am on the day of the survey were included, and data were collected by clinical pharmacists in each of the hospital wards on the prevalence and patterns of antimicrobial use quantity as well as quality indicators of the prescriptions via patients’ case notes. Where such information was not documented in case notes, it was obtained by direct inquiry to the medical or nursing staff on the ward. Guidelines for completing the survey data were distributed and clinical pharmacists also met to address any data collection issues. On the day of the PPS, data were gathered on the number of inpatients in each department (denominator), age, gender, medical specialty, antimicrobials used, dose and route, as well as anatomic infection site.
The antimicrobials classification system used was the World Health Organization's (WHO) Anatomic Therapeutic Chemical (ATC) classification (www.whocc.no/atc_ddd_index). Quality indicators included the indication for antimicrobial treatment, hospital-acquired infections defined as signs/symptoms of infections occurring >48 h after admission to hospital, community-acquired infections receiving antibiotics or signs/symptoms <48 h after admission to hospital and prophylaxis (medical and surgical). Compliance with the hospital's prescribing policy against standard guidelines regarding antibiotic choice (route, dose, duration) and classed as compliant, non-compliant, not assessable where the diagnosis was an unclear or unknown indication. The use of a biomarker of infection (e.g. C reactive protein) to inform antibiotic treatment was noted. Further information on the definitions used in the Global-PPS protocol is available online (www.global-pps.com/documents). The prevalence of antibiotic prescription was calculated by dividing the number of patients treated with an antibiotic over the total number of inpatients surveyed.
The χ 2 test was used to assess the differences in quality indicators over the three studies. The statistical significance level was set at P = 0.05. SPSS version 21 software was used for the statistical analysis.
Results
In total, 3605 inpatients were surveyed in the three hospitals over the three time periods (2009, 2011 and 2015): 1203 patients in May 2009, 876 in June 2011 and 1526 in May 2015, respectively. Table 1 summarises the general characteristics and antibiotic prescribing patterns of the patients surveyed.
Table 1. General characteristics and antibiotic prescription patterns of patients surveyed at three time points (2009, 2011 and 2015)
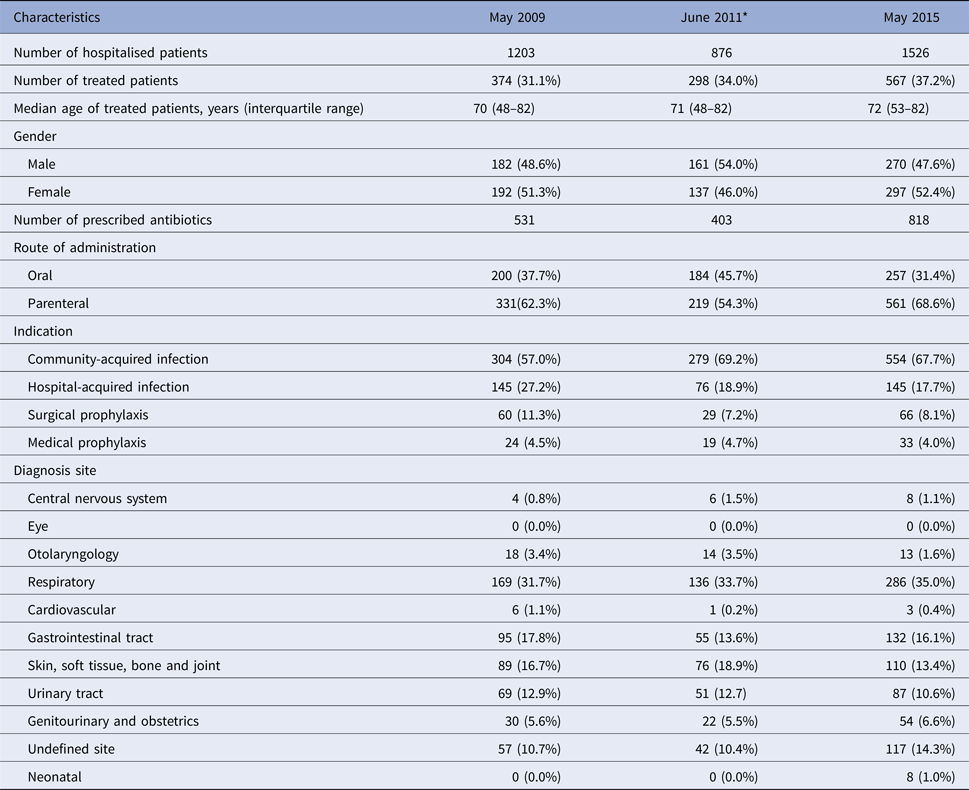
* Data were collected from two hospitals: Antrim Area Hospital and Ulster Hospital.
Of all 3605 patients surveyed, 1239 (34.4%) were treated with an antibiotic; approximately 60% were prescribed parenteral antibiotics and the remainder oral antibiotics. Several trends related to the number and types of antibiotics prescribed were noted across the three time points. Overall, one-third of patients surveyed were treated with an antimicrobial and this proportion increased slightly over time, e.g. 31.1% in 2009, 34.0% in June 2011 and 37.2% in May 2015. In 2009, 374 patients received a total of 531 antimicrobials (200 oral and 331 parenteral), 298 in 2011 with 403 antimicrobials (184 oral and 219 parenteral) and 567 in 2015 with 818 antimicrobials (257 oral and 561 parenteral). Across all time points, on average, 64.6% of patients were prescribed antimicrobials for a community-acquired infection, 21.3% for hospital-acquired infections, 8.9% received antimicrobials for surgical prophylaxis and 4.4% for medical prophylaxis. The most frequent infection sites, on average, were respiratory (33.5%), skin and soft tissue and bone and joint (16.3%) and gastrointestinal sites (15.8%).
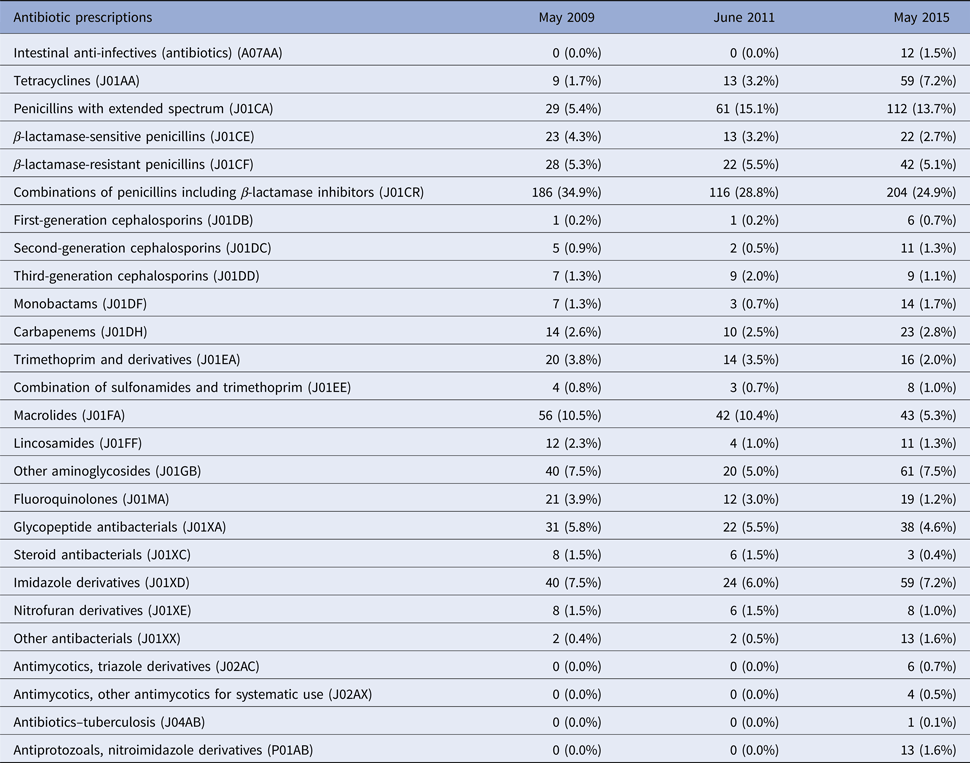
Table 2 shows that the most frequently prescribed antibiotic groups at the three time points were a combination of penicillins, including β-lactamase inhibitors accounting for 34.9%, 28.8% and 24.9% of prescriptions in 2009, 2011 and 2015, respectively. In 2009 and 2011, macrolides represented the second most frequent group (10.5% and 10.4% in 2009 and 2011) but decreased by half (5.3%) in 2015. Penicillins with extended spectrum were prescribed for 15.1% and 13.7% of patients in 2011 and 2015, compared with 5.4% in 2009. Other notable trends included an increase in the prescription rate for tetracyclines; 7.2% in 2015 vs. 1.7% and 3.2% in 2009 and 2011. Likewise, fluoroquinolones (J01MA) usage declined to 1.2% in 2015 from 3.9% and 3.0% in 2009 and 2011, respectively.
Table 2. Antibiotic agents prescribed at three time points (2009, 2011 and 2015)
Over the three time periods, a general trend in the improvement of quality indices (compliance with antibiotic guidelines and duration of surgical prophylaxis) – with some exceptions – was noted (Table 3). For example, 54.5% compliance to antibiotic guidelines was reported in 2009, 71.5% in 2011 and 79.9% in 2015 (P < 0.001). Other indicators, i.e. treatment based on biomarker data, were used in 61.5% of cases (May 2015).
Table 3. Quality indicators at three time points (2009, 2011 and 2015) in the study hospitals

a One patient's data are missing.
b Includes data collected from Craigavon Area Hospital in Southern Health and Social Care Trust (SHSCT) in February 2012.
c This figure includes data from Altnagelvin Hospital in Western Health and Social Care Trust (WHSCT).
Discussion
The present study aimed to evaluate the prescribing patterns of antimicrobials and quantify progress in relation to targets for quality improvement in the prescription of antimicrobials in Northern Ireland's secondary care sector using three repetitive PPS over a 6-year period.
Improvements were identified in key antimicrobial-related quality-of-service outcomes and attributed to evidence-based, clinical pharmacist-led, antimicrobial stewardship programmes in the study hospitals. Areas for further work were also identified. Good outcomes with antimicrobials (i.e. appropriate antimicrobial prescribing and reduction of resistance to antimicrobials) require the use of antimicrobial stewardship approaches and completion of PPS at regular intervals [Reference Willemsen10, Reference Retamar11]. We used PPS data collected at three time points (2009, 2011 and 2015) in three major hospitals in Northern Ireland. A PPS served as a convenient, inexpensive surveillance system of antimicrobial consumption, as opposed to continuous surveillance. The number of patients in our study increased in time, as did the proportion treated with an antimicrobial. Such a trend is a challenge to the hospitals surveyed and indicates that increasing efforts are needed from hospital staff. The finding that approximately one-third of patients were prescribed antimicrobials is consistent with previously published studies [Reference Robert2, Reference Skoog12–Reference Štefkovičová15].
A number of studies have assessed the relationship between differences in prescribing patterns and the occurrence of resistance in bacterial isolates and for the basis for the common practice of restriction of use and cycling of prescribed antibiotics to combat the emergence of antibiotic resistance [Reference Goossens5, Reference Costelloe6, Reference Bantar16]. Based on an ecological study in Europe, countries with higher antibiotic consumption had correspondingly higher rates of antimicrobial resistance, underlining the fact that uncontrolled prescribing of antibiotics is a key risk factor for resistance [Reference Goossens5]. Further support for this link was evident by the decrease of resistance with reduced prescribing of broad-spectrum antibiotics, which was also associated with a cost-saving outcome [Reference Bantar16]. Similar findings were reported by others for antibiotic prescriptions for urinary and respiratory tracts infection [Reference Costelloe6]. Many factors can lead to fluctuations and misuse in antibiotic use, such as overprescription of broad-spectrum antibiotics and inappropriate treatment of likely viral respiratory tract infections [Reference Wise17]. Indeed, the lack of prescriber awareness of the impact of antibiotics on the emergence of resistance remains a key driver of inappropriate antimicrobial use and rates of resistance [Reference Costelloe6].
The majority of patients surveyed here were middle-aged (45–65 years) or greater. Across the three time points, consistent results of general patient characteristics were evident, particularly in relation to age, thereby highlighting the utility of comparing patterns of antimicrobial use across different time points; these patterns were consistent in prescription rates for most antimicrobials at the different time points. However, antibiotic policies within the study hospitals, together with previous PPS, have resulted in a decrease in the utilisation of certain antimicrobials, especially macrolides (including clarithromycin) and fluoroquinolones. Antimicrobial policies are updated annually to take into account local issues and resistance patterns. In the NHSCT, from 2013 to 2015, there was a policy to assess the impact of cycling antibiotics on the prevalence of bacterial resistance, which resulted in the restriction of certain antibiotics at different time periods and on awareness of the policy and their necessary documentation (posters/leaflets, etc.) to promote adherence to the new policy. In the SEHSCT, a smartphone application to disseminate the guidelines was introduced in October 2014. Moreover, the empirical first-line drug guidelines (in response to the PPS results) were amended to reduce specifically macrolide use, e.g. doxycycline replaced clarithromycin as a penicillin-allergic option for community-acquired pneumonia in the guideline.
Other broad-spectrum antibiotic use is also expected to have decreased due to antimicrobial stewardship and restriction of their use. This has led to an increase in the use of tetracyclines and penicillins with extended spectrum, as identified in the present study. Similar trends were noted by others as prescriptions for quinolones decreased by half in repeated PPS in Sweden [Reference Skoog12]. In Northern Ireland, input from initial point prevalence survey in 2008 was used to inform antimicrobial stewardship interventions, that resulted in reduction in prescribing of amoxicillin-clavulanic acid in 2009 [Reference Aldeyab18]. Our study is therefore consistent with the hypothesis that repeated PPS allow the identification of targets for quality control relevant to the prescribing of antimicrobials. The AMT sets targets within each hospital Trust covering adherence to guidelines and documentation of core data, which were subject to continuous audit with the results being communicated at monthly antimicrobial management meetings to clinical consultants, ward managers and pharmacists.
In the NHSCT, multidisciplinary rounds were undertaken in wards where antibiotic use was the highest use. The SEHSCT Trust developed guidelines for certain patient care units, such as intensive care through collaboration with clinical pharmacists’ specialists taking the lead, medical practitioners and microbiologists, which led to documented improvements in the quality of services. However, the increased compliance to guidelines should be interpreted with caution, as it may be due, in part, to increased level (90%) of documentation of indications for treatment. Moreover, compliance with guidelines and documentation is audited on routine microbiology stewardship rounds and annual review by the microbiologists and antimicrobial pharmacists to identify gaps and resolve anomalies.
Consistent improvement in the duration of surgical prophylaxis was obtained over the three time points in the study from 3.9% in 2009 to 0.7% in 2015 (Table 3; P < 0.001). This was a direct result of the combination of antimicrobial stewardship, antibiotic policies, previous PPS leading to increased education and awareness of the importance of single-dose surgical prophylaxis. In the NHSCT, there is a separate prescription and administration record for surgery distinct from the main medication record; this reduces the incidence of omission of entry of details of surgical prophylaxis. The limited number of surgical disciplines enables closer engagement with them on guideline development and review; the choice of antimicrobials was minimised to aid the selection of appropriate prophylaxis by surgical staff. For example, in SEHSCT, cefuroxime, which was recommended for general surgical prophylaxis, was removed from all adult wards and stocked only in operating theatres. Currently, a daily electronic report of all orders for this antibiotic from general wards is reviewed by the antimicrobial pharmacy team. Prolonged surgical prophylaxis may contribute to increased antimicrobial resistance, and evidence shows that single-dose prophylaxis is effective. Despite this, there is inconsistency in practice with some favouring the use of prolonged courses [Reference Hohmann19–Reference Bratzler and Houck21].
The limitations of this study are inherent with cross-sectional surveys of this type. Data were not corrected for comorbidity or other patient characteristics affecting antibiotic prescribing and did not obtain information about the clinical justification of the infection to be treated and the duration of the antibiotic therapy; both are also important quality indicators of antibiotic prescribing. The observed improvement in guideline compliance might be related to increased documentation of indication. Despite this, the overall dynamics suggest that there is a consistent improvement in the quality of prescribing with respect to the type of antibiotic, compliance with guidelines and documentation of the prescription.
The present findings have important clinical implications as evident in the longitudinal improvements in quality indices and changes in antimicrobial prescribing patterns. Other indices such as biomarker data were identified in 2015 as targets for quality improvements, which can serve to guide appropriate antibiotic treatment and may reduce unnecessary antibiotic use as seen with macrolides. It is clear that improving antibiotic prescribing practices contribute to reducing antibiotic use, and should lead to a reduction in healthcare-acquired infections [Reference Aldeyab22]. Of note, PPS allows hospitals to benchmark their antibiotic use, internally and externally, leading to better utilisation of antibiotics and best clinical practices [Reference Aldeyab23].
In conclusion, this PPS study of longitudinal trends in antimicrobial use highlighted the impact of the antimicrobial policies within the hospital and the role of antimicrobial stewardship, as noted in the reduction of the prescribing of certain antimicrobials (e.g. fluoroquinolones).
Acknowledgements
All clinical pharmacists, in each participating Trust, for their help in data collection for this study






