Bacteraemia is among the leading causes of global childhood deaths [Reference Kanoksil1]. It is common in children with severe acute malnutrition (SAM) [Reference Nhampossa2], with incidence rates as high as 29% in those with pneumonia with 23% deaths [Reference Chisti3]. The comorbidity of SAM and pneumonia is very common, especially in developing countries [Reference Chisti3] and clinicians in resource-poor settings mostly rely on clinical features of bacteraemia for the timely management of such children [Reference Chisti4]. However, the clinical signs of bacteraemia in SAM children are often subtle [Reference Chisti3], probably due to poor inflammatory response [Reference Walker5] and overlapping clinical signs of pneumonia [6]. Such children are often treated with the combination of injectable penicillin/ampicillin and gentamicin [6]. Although, this combination is very effective in treating children with severe pneumonia [Reference Asghar7], bacteraemia in these cases are due to Gram-negative bacteria [Reference Chisti8] which may be multiply drug resistant [Reference Chisti4]. SAM children with pneumonia and bacteraemia require aggressive antimicrobial therapy, using a third-generation cephalosporin (ceftriaxone) in addition to other supportive measures to prevent death [Reference Ahmed9]. Failure to recognize bacteraemia may result in their treatment with standard regimens of penicillin/ampicillin and gentamicin with poor outcomes [Reference Blomberg10]. Therefore, identification of clinical characteristics and common pathogens causing bacteraemia along with the knowledge of their antimicrobial susceptibility are important for the treatment of SAM children with pneumonia. Further, there are limited data on clinical risks of bacteraemia in SAM children with pneumonia.
The Dhaka Hospital of the International Centre for Diarrhoeal Disease Research, Bangladesh (ICDDR,B) provides care and treatment to large numbers of SAM children with pneumonia aged <5 years each year, who often have bacteraemia with high case fatality [Reference Chisti8]. The aim of our study was to identify clinical predictors of bacteraemia in SAM children with pneumonia and the outcome of such children.
This study was approved by the Institutional Review Board of ICDDR,B (known as the Research Review Committee and Ethical Review Committee). Written informed consent was obtained from attending parents or caregivers of each of the participating children. The study was designed as an unmatched case-control investigation. Severe malnutrition was defined according to WHO anthropometry as described elsewhere [Reference Chisti11]. SAM children of both sexes, aged 0–59 months, admitted for intensive or high dependency care unit with radiologically confirmed pneumonia (WHO criteria [6]) during April 2011 to July 2012 were enrolled. Those with bacteraemia, defined as isolation of bacteria from a single specimen of blood culture on admission, constituted the cases and those without bacteraemia were used as controls.
Venous blood (2–5 ml) was taken from all study children before starting antibiotics, and inoculated into standard paediatric blood culture bottles and processed for bacterial culture, species identification and antimicrobial susceptibility as previously described [Reference Chisti4].
Antibiotic and supportive therapy was as described elsewhere [Reference Gilman12] and all study children received on admittance to ICDDR,B diphtheria-pertussis-tetanus (DPT), oral polio, Haemophilus influenzae b vaccine (Hib), hepatitis, measles, and bacillus Calmette-Guérin (BCG) vaccination as recommended by the WHO [13].
Case report forms were developed, pretested, and finalized for data acquisition. Characteristics analysed include demographic and medical history (age, gender, socioeconomic status, lack of previous vaccinations, non-breastfeeding, prior antibiotics before admission), clinical signs such as acute watery diarrhoea (AWD), vomiting, and dehydration according to the ‘Dhaka methods’ of assessment of dehydration approved by WHO [Reference Alam and Ashraf14]. Other measurements included fever (⩾38°C), hypoxaemia (SPO2 <90% in air), diastolic hypotension, hypoglycaemia (blood sugar <3 mmol/l), pulmonary tuberculosis, and outcome.
All data were entered into SPSS for Windows version 15.0 (SPSS Inc., USA) and Epi-Info version 6.0 (USD, USA). Proportional differences were compared by χ 2 test. For normally distributed data, differences of means were compared by Student's t test and for non-normal distributions, the Mann–Whitney test was used. P ⩽ 0·05 was considered statistically significant. Strength of association was determined by odds ratio (OR) and 95% confidence intervals (CIs). For identifying predictors of bacteraemia in children with SAM and pneumonia, variables were initially analysed in a univariate model, and independent predictors were identified using a logistic regression model; significantly associated variables with bacteraemia by univariate model were considered as independent variables and bacteraemia as a dependent variable.
In total, 405 children were enrolled according to the study criteria giving 18 cases and 387 controls. Of the cases, two each yielded two different bacterial species from the same blood culture, and overall 11 species groups were identified. Streptococcus pneumoniae was the most common species isolated (four cases) but Gram-negative species accounted for 75% of all isolates recovered and included Klebsiella spp. (n = 3), Salmonella spp. (n = 3, two S. typhi). Pseudomonas spp. (n = 2), and Acinetobacter spp. (n = 2), among others. Antimicrobial susceptibility was variable across the range of species.
The death rate was significantly higher in cases (28%) than controls (8%) and of the children that died, two grew Klebseilla spp., one each S. pneumoniae, Pseudomonas spp., and Acinetobacter spp. On the basis of antimicrobial susceptibility test results of these isolates, the patients with S. pneumoniae and Acinetobacter bacteraemia had received appropriate antibiotics; for the Klebseilla spp. cases one had received appropriate and the other inappropriate antibiotics, and similarly the single Pseudomonas case had received antibiotics to which the isolate was resistant. Susceptibility reports were received after the death of the patients who had received inappropriate antibiotics.
The distribution of gender, age, socioeconomic condition, breastfeeding, use of antibiotics before admission, AWD, fever, hypoxaemia, and pulmonary tuberculosis was comparable among cases and controls. However, significant differences from controls were identified in the cases for vomiting, clinical dehydration, lower Z score (weight for length), and hypoglycaemia (Table 1). Moreover, in the logistic regression analysis after adjusting for potential confounders listed above, cases more often had a history of lack of BCG vaccination (OR 7·39, 95% CI 1·67–32·73, P < 0·01).
Table 1. Clinical characteristics of children aged <5 years having pneumonia and severe acute malnutrition with (cases) and without (controls) bacteraemia
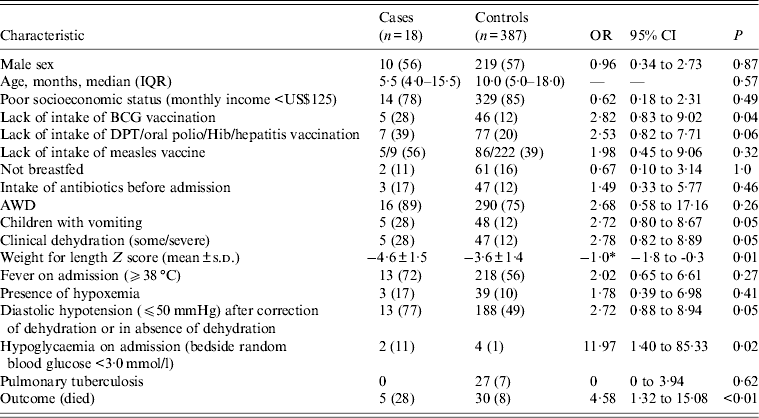
OR, Odds ratio; CI, confidence interval; IQR, interquartile range; BCG, bacillus Calmette-Guérin; AWD, acute watery diarrhoea; s.d., standard deviation.
Figures represent n (%), unless specified otherwise.
* Mean difference.
To our knowledge this is the only study performed outside of Africa that has evaluated the presence of bacteraemia in severely malnourished (SAM) children with pneumonia. The main findings are (i) death rates were significantly higher in those patients with pneumonia and bacteraemia compared to those without bacteraemia, (ii) a wide range of Gram-negative species was evident and these were most associated with mortality, and (iii) there was a strong association of bacteraemia with a lack of BCG vaccination. The predominance of Gram-negative species has been noted previously [Reference Nhampossa2] but the factors underlying the observation are poorly understood. The oropharynx in severely malnourished children is often colonized with enteric bacteria that can potentially spread to the lower respiratory tract with or without additional factors such as viral respiratory tract infections [Reference Gilman12]. However, we did not perform nasopharyngeal aspirate culture in cases that may demonstrate the same species as in blood and may help in validating the speculation. Breaches in the integrity of the bowel mucosa as well as translocation of bacteria from the gut might lead to Gram-negative bacteraemia in children with SAM and pneumonia [Reference Chisti8] and warrant aggressive management with appropriate broad-spectrum parenteral antibiotics.
The key observation made here of a strongly significant association between a lack of BCG vaccination and bacteraemia is of profound importance for clinicians and policymakers, especially in developing countries. BCG vaccination against tuberculosis has been associated with non-tubercular beneficial effects [Reference Shann15] and has been reported to reduce ~50% of deaths from infections other than tuberculosis, such as pneumonia-related deaths in high childhood mortality countries in the developing world [Reference Shann16]. However, a lack of BCG vaccination as an independent predictor of bacteraemia in malnourished children aged <5 years has not hitherto been reported. This may be a substantial heterologous effect of BCG in children and suggests its potential for reducing the incidence of bacteraemia in tuberculosis-endemic countries.
A limitation of the study is the lack of data on airways/secretions culture in cases. Demonstration of the same species in such specimens and blood would have been informative.
In conclusion, we have found that SAM children with pneumonia often present with Gram-negative bacteraemia. Infection in these children appears to be strongly associated with a history of lack of BCG vaccination. This finding underscores the importance of the continuation of BCG vaccination in children to attain the non-tubercular substantial benefit of the vaccination.
ACKNOWLEDGEMENTS
This research was funded by ICDDR,B and its donors who provide unrestricted support to the institute for its operations and research. Current donors include: Australian Agency for International Development (AusAID), Government of the People's Republic of Bangladesh, Canadian International Development Agency (CIDA), Swedish International Development Cooperation Agency (Sida), and the Department for International Development, UK (DFID). We gratefully acknowledge these donors for their support and commitment to ICDDR,B research efforts. We express our sincere thanks to all physicians, clinical fellows, nurses, members of feeding team and cleaners of the hospital for their invaluable support and contribution during patient enrolment and data collection. We also express our gratitude to the caregivers/mothers of the study participants for their consent to enrol their children in the study.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
DECLARATION OF INTEREST
None.




