INTRODUCTION
Hand, foot and mouth disease (HFMD) is a common childhood infection, especially for those aged <5 years. The disease usually presents with rashes over fingers and palms, feet and other parts of the body such as buttocks and thighs. Vesicles can be found on the tongue and soft palate. In most cases, the illnesses are self-limiting and patients can recover completely. However, some patients may develop severe complications including meningitis, encephalitis, acute flaccid paralysis, myocariditis, pulmonary oedema, or even death, especially when the illness was caused by enterovirus 71 [Reference Frydenberg and Starr1, Reference Kushner and Caldwell2]. HFMD is mainly transmitted by the faecal–oral route and respiratory droplets. The infection is caused by various serotypes of enteroviruses. The most common cause of HFMD is coxsackie virus A16 while other types of enteroviruses have also been associated with this syndrome, such as coxasackie viruses A4, A5, A9, A10, B2, B5, and enterovirus 71. Direct contact with open and weeping skin vesicles or contaminated objects may also transmit the viruses. The viruses can also be excreted in stools of infected patients for several weeks and can survive for days on fomites at room temperature.
Epidemics of HFMD have been reported worldwide since the 1970s in Bulgaria, Hungary, UK, USA, Australia, and India with significant mortality and morbidity [Reference Ho3–Reference Gilbert7]. Large outbreaks due to enterovirus 71 have occurred from 1997 to 2000 in Malaysia, Taiwan, Singapore, and Japan [Reference AbuBakar8–Reference Fujimoto11]. Over 100 000 cases of HFMD including 400 severe illnesses and 78 deaths were reported in Taiwan in 1999 [Reference Ho9]. In 2008, another epidemic affected the South East Asia region including Mainland China, Taiwan, Singapore, and Hong Kong [Reference Ding12–Reference Ma15]. The cyclical rise in HFMD activity has attracted much international attention and hence health authorities need to closely monitor the disease's activity through various surveillance systems.
Associations between climate factors and various infections, particularly influenza, have been studied [Reference Lowen16–Reference Chan19]. Chan et al. have demonstrated that cold and humid conditions are associated with a higher activity of both influenza A and B in Hong Kong [Reference Chan19]. However, little is known regarding this aspect for HFMD. The objective of the present study was to examine the relationship between climate change as measured by various meteorological parameters and HFMD activity as reflected by the consultation rates of HFMD diagnosed by general practitioners (GPs) participating in the sentinel surveillance system in Hong Kong. Through this study, we hope to better understand the epidemiology of HFMD, which in turn will assist in formulating preventive strategies.
METHODS
Study methods
This was a retrospective study examining the relationship between meteorological parameters and HFMD disease activity in the community as indicated by HMFD consultation rates calculated through the sentinel surveillance system. The data collected in the past decade (2000–2009), were divided into two study periods. For the first half of the study period, we examined the data collected from 2000 to 2004 to explore whether there was any association between meteorological parameters and HFMD consultation rates, and developed the model explaining the trend of consultations. For the second period from 2005 to 2009, we used the established model to predict HFMD consultation rates, and compared the observed and the predicted rates.
HFMD activity
HFMD is a common paediatric disease encountered by GPs in Hong Kong. The sentinel surveillance system is a well established system developed by the Department of Health to monitor various infectious diseases including HFMD. There are around 40 GPs widely distributed throughout the territory participating in the surveillance system. Every week, these GPs record the number of HFMD cases seen in the past 7 days. A HFMD case was defined as a patient having symptoms compatible with HFMD with or without laboratory confirmation. Common presentations of HFMD were fever, sore throat, skin rash over hands and feet, and vesicles in the oral cavity, on the tongue and palate. The HFMD consultation rate was calculated by first summing up the total number of HFMD cases seen by all sentinel doctors and then divided by the total number of consultations of these doctors. This weekly consultation rate was expressed as the number of HFMD cases per 1000 consultations.
Meteorological parameters
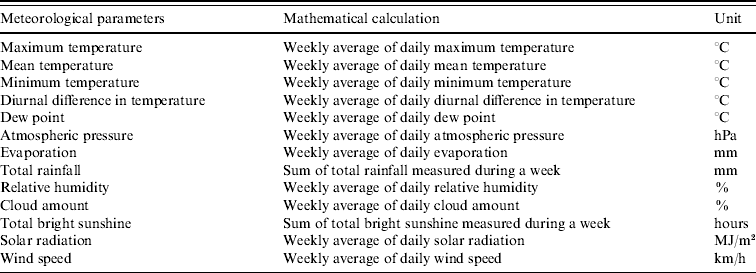
The meteorological data collected during 2000–2009 were obtained from the Hong Kong Observatory. These included mean temperature, relative humidity, total rainfall, atmospheric pressure, wind speed, solar radiation and other parameters. Daily diurnal variation in temperature was calculated by subtracting the maximum and minimum temperature. All the meteorological parameters studied and the values used for testing the associations are summarized in the Appendix. For example, the weekly mean of maximum temperature was calculated by averaging the daily maximum temperature of a week, while total rainfall was calculated by summing up the amount of rainfall measured for the whole week.
Data analysis
We examined for any association between HFMD consultation rates and each meteorological parameter using Spearman's rank correlation. Since different meteorological parameters might be correlated with each other, we further tested for any correlation among the meteorological parameters using Pearson's or Spearman's rank correlation whichever was appropriate. Including two strongly collinear independent variables in a regression model may potentially lead to an erroneous conclusion that there is no association with the outcome variable even if in fact there is [Reference Næs and Mevik20]. This occurs when collinearity is sufficiently high to dramatically increase the standard errors of the coefficients. In order to adjust for any effect of correlation between meteorological parameters on HFMD activity, the meteorological parameters were entered into the multiple linear regression model. A forward stepwise approach was used in the regression model by adding meteorological parameter step by step until the best-fit model was found. We reported the partial correlation coefficient and the 95% confidence interval (upper and lower limits) of each parameter in the model. Furthermore, theoretically, if HFMD activity was indeed affected by the change in climate conditions, the change in HFMD consultation rates should lag behind the meteorological parameters, taking into account the incubation period of HFMD and the delay in seeking medical attention. To adjust for this, we repeated the regression analysis using HFMD data with 1, 2 and 3 weeks lag time, assuming that the incubation period for coxsackie viruses (common pathogens for causing HFMD), were about 1 week's duration [Reference Heymann21]. During the severe acute respiratory syndrome (SARS) epidemic in 2003, transmission of respiratory viruses in Hong Kong was greatly reduced by massive use of face masks and school closures [Reference Lo22]. We also performed the regression analysis after excluding the data collected during the SARS period. SPSS software, version 14.0 (SPSS Inc., USA) was used for analysis. Two-tailed analysis was used for all statistical tests and P values <0·05 were considered to be statistically significant.
In the second half of the study, the model constructed from the above analysis was used to predict the HFMD consultation rates from 2005 to 2009. We compared the actual and predicted values of HFMD consultation rates using Spearman's rank correlation. The prediction was repeated after excluding the data collected during pandemic influenza H1N1 in summer 2009, due to similar reasons mentioned above for the SARS period. We also performed sensitivity analysis in order to examine how HFMD consultation rates varied if we changed the estimates of the partial correlation coefficient of the meteorological parameters, using the upper and lower limits found in the regression model.
RESULTS
The trend of the HFMD consultation rate from 2000 to 2009 is shown in Figure 1. It was found that a seasonal peak occurred for the summer months of each year except in 2003 and 2009 when SARS and pandemic influenza H1N1 occurred, respectively. Interestingly, a smaller winter peak was also noted in the last 12 weeks (around October–December) of each year since 2006.

Fig. 1. Weekly consultation rates of hand, foot and mouth disease (HFMD) detected by the sentinel surveillance system based at general practitioners in Hong Kong, 2000–2009.
For the individual meteorological parameter, HFMD consultation rates were positively associated with maximum temperature, mean temperature, minimum temperature, diurnal difference in temperature, dew point, relative humidity, evaporation and total rainfall, and were negatively associated with atmospheric pressure (Table 1). Of these factors, maximum temperature, dew point and atmospheric pressure had the strongest association with Spearman's rank correlation coefficient ranging from 0·261 to 0·334. Total sunshine and wind speed were not significantly associated with HFMD consultation rates.
Table 1. Spearman's rank correlation between various meteorological factors and hand, foot and mouth disease consultation rates detected by sentinel surveillance system, 2000–2004
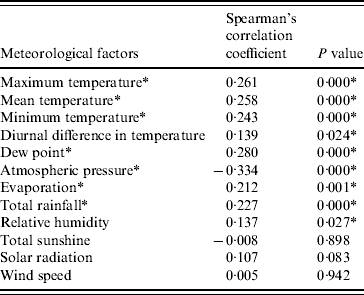
* Statistically significant.
Various meteorological parameters were found to be inter-related with each other. Table 2 summarized the correlation coefficients between all these parameters. It was found that mean temperature, maximum temperature, minimum temperature, dew point, atmospheric pressure and evaporation were highly correlated with each other, with the correlation coefficient >0·80. Moreover, total bright sunshine and solar radiation were also highly inter-related with a correlation coefficient of 0·88. Hence, we included only mean temperature and solar radiation in the regression analysis model, and excluded other highly correlated variables. Apart from these two variables, the other variables included in the regression analysis were relative humidity, diurnal difference in temperature, total rainfall, and wind speed.
Table 2. Correlation coefficient between different meteorological parameters measured from 2000–2004
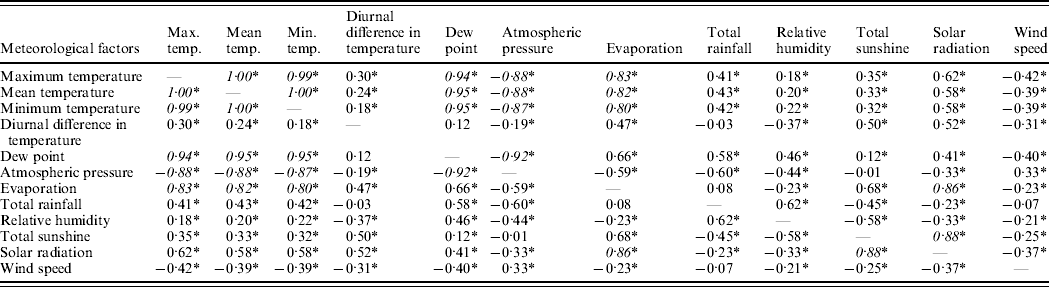
Italicized values are ⩾0·80 indicating the two factors are highly correlated with each other.
Either Pearson's or Spearman's rank correlation are used as appropriate.
* P value <0·05.
Table 3 shows the results of linear regression analysis to explain the HFMD consultation rates during 2000–2004. In the M0 model, which had no lag time for analysing HFMD consultation rates and climate parameters, HFMD consultation rates were shown to be positively associated with mean temperature and rainfall, after adjusting for the effect of other parameters. When the lag time was taken into consideration, the M2 model was a better fit, with a higher R 2 value of 0·119 than the M0 and M1 models which had R 2 values of 0·079 and 0·062 respectively. This indicated that HFMD consultation rates were better explained using meteorological parameters measured 2 weeks earlier. In the M2 model, mean temperature, diurnal difference in temperature, relative humidity, and wind speed were positively associated with HFMD consultation rate. The R 2 value of the M2 model would be even higher (0·154) if we excluded the SARS period in 2003. The M3 model showed similar results with a slightly higher R 2 of 0·122.
Table 3. Linear regression models using various meteorological parameters to explain hand, foot and mouth disease consultation rates, 2000–2004
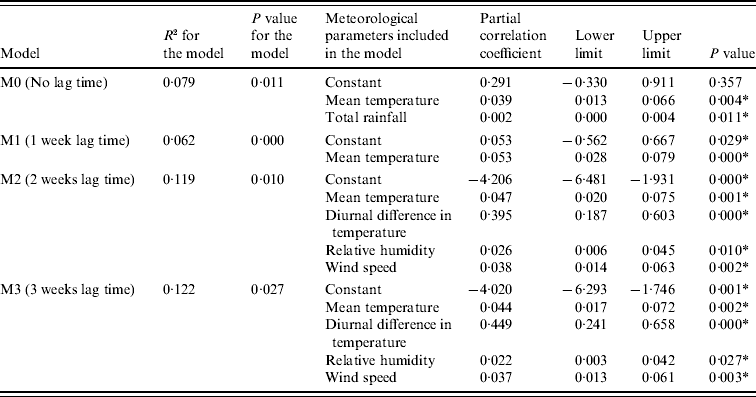
* Statistically significant.
Taking into account that the incubation period for enteroviruses causing HFMD was about 1 week and assuming that patients sought medical consultation a couple of days after clinical presentation, we estimated the lag time between climate parameters and HFMD consultation rates to be ~2 weeks. In addition, the R 2 of the M3 model was actually more or less the same as that of the M2 model. Hence, we used the M2 model for our prediction of HFMD consultation rates for the years 2005–2009. The predicted trend of HFMD consultation rates matched quite well with the observed trend, with Spearman's rank correlation coefficient being 0·276, P=0·000 (Fig. 2). However, it was noted in the summer months of 2009 when pandemic influenza H1N1 occurred, that the observed HFMD consultation rates were lower than the predicted rates. If the data during this period were excluded, we would obtain a better correlation (Spearman's rank correlation coefficient of 0·298 vs. 0·276) between the predicted and the observed HFMD consultation rates. The results of the sensitivity analysis are shown in Figure 3(a–d) illustrating how the estimated HFMD consultation rates change if we varied the partial coefficients of mean temperature, diurnal difference in temperature, relative humidity, and wind speed. It was noted that HFMD consultation rates were mostly affected by varying the relative humidity while they were least affected by wind speed.
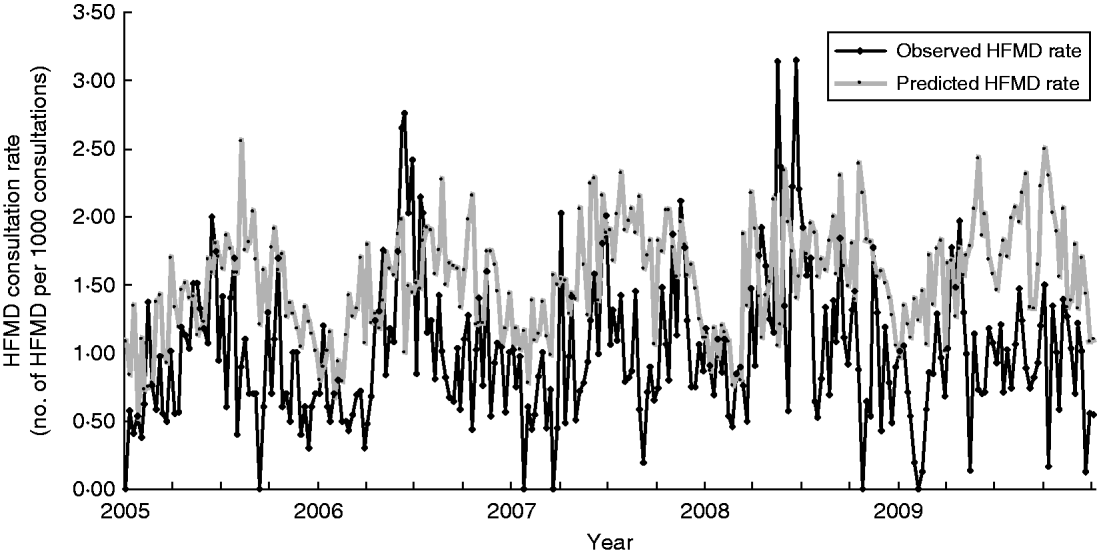
Fig. 2. Observed and predicted weekly hand, foot and mouth disease (HFMD) consultation rates, 2005–2009.

Fig. 3. Sensitivity analysis of predicted hand, foot and mouth disease (HFMD) consultation rates by varying partial correlation coefficients of (a) mean temperature, (b) diurnal difference in temperature, (c) relative humidity and (d) wind speed while adjusting the other meteorological parameters. LL, Lower limit; UL, upper limit.
DISCUSSION
Although non-polio enteroviruses are distributed worldwide, their prevalence varies with time and place. In countries with temperate climates, epidemics tend to occur in the summer and autumn months while infections are common throughout the year in tropical regions [Reference Bendig and Fleming4, 6, Reference Druyts-Voets23–Reference Podin26]. Hong Kong is situated at latitude of 22·5° North with a sub-tropical, tending towards temperate, climate for almost half the year. It is hot and humid in the summer months and afternoon temperatures often exceed 31°C whereas at night, temperatures generally remain around 26°C with high humidity [27]. The seasonality of HFMD detected by the sentinel surveillance system in Hong Kong is similar to the patterns seen in other areas of the region [Reference Chen24–Reference Podin26]. The present study shows HFMD activity is significantly associated with different meteorological parameters. Increasing the temperature and relative humidity would increase HFMD activity while increasing the atmospheric pressure would decrease its activity. The mean temperature, diurnal difference in temperature, relative humidity, and wind speed are the most important factors after adjusting the correlation between meteorological parameters. The HFMD trends are better explained if we use meteorological parameters measured 2 weeks before the HFMD clinical consultation rates reported to the health authority. This is compatible with an understanding of the incubation period and possible delay in seeking medical care by the patients. In Hong Kong, most of the viruses causing HFMD are coxsackie A16, and occasionally other coxsackie A subtypes, coxsackie B viruses or enterovirus 71. The incubation period for these enteroviruses is about 3–5 days [Reference Heymann21]. This explains why the M2 model has a higher R 2 value than the M0 or M1 models.
There are plenty of studies that have investigated the relationship between climate factors and various infectious diseases [Reference Lowen16–Reference Chan19, Reference Zuk, Rakowski and Radomski28–Reference Zuk, Rakowski and Radomski30]. The most intensively studied is seasonal influenza. For example, Zuk et al. demonstrated that spread of influenza virus depends on various temperature and relative air humidity levels [Reference Zuk, Rakowski and Radomski28]. On the other hand, there is only limited literature that identifies this aspect for HFMD. Nevertheless, the fact that HFMD occurs more commonly in temperate regions and that the seasonality detected by different countries suggests climate factors may have an important role in determining the activity of enteroviruses. In a Japanese study examining the relationship of weather conditions and HFMD and herpangina cases detected at sentinel paediatric clinics, it was shown that higher air temperature and humidity/vapour pressure, and lower precipitation and duration of sunshine increased the incidence of HFMD or herpangina [Reference Urashima, Shindo and Okabe31]. This is similar to our findings although total rainfall (precipitation) and duration of sunshine or solar radiation were not significantly associated with HFMD in our M2 regression model.
There are several postulations on how meteorological factors affect viral transmission [Reference Abad, Pintó and Bosch32–Reference McGeady, Siak and Crowell34]. Laboratory studies have shown the stability of enteric viruses are influenced by environmental factors such as temperature and relative humidity [Reference Abad, Pintó and Bosch32–Reference McGeady, Siak and Crowell34]. The viruses may have a more rapid decline in activity during dry seasons [Reference Abad, Pintó and Bosch32]. Ud-Dean postulated that the viral envelope of influenza determines its persistence and transmission in various environmental conditions [Reference Minhaz Ud-Dean35]. Whether the same theory applies to enteroviruses needs further experimental studies. On the other hand, host behaviour may also differ in different seasons [Reference Dowell36]. For example, children are more likely to go outdoors to playgrounds during summer than in winter when it is cold and windy. This may in turn facilitate transmission of enteroviruses through respiratory droplets (when children have close contact), open and weeping skin vesicles, or direct contact of contaminated toys and environmental surfaces. In the M2 model, the HFMD consultation rate is also associated with diurnal difference in daily temperature and wind speed. Higher wind speed may favour the spread of disease through respiratory droplets. The exact mechanism of how diurnal difference in temperature operates is not well understood. It may act through the diminishing immunity of the host or by directly affecting viral growth.
It should be noted that apart from climatic parameters, HFMD activity may also be influenced by other factors. Possible contributing factors include immunity of the susceptible population, prevalence of different enteroviruses circulating in the community, and public health measures implemented [6, Reference Podin26]. For example, during the 2003 SARS epidemic in Hong Kong and the 2009 H1N1 influenza pandemic, there were territory-wide school closures. Such social distancing measures and intensive education on use of face masks helped to reduce disease transmission [Reference Lo22]. This explains why the observed HFMD rates during summer 2009 are lower than the predicted rates in the projection analysis.
Climate change has been considered as the biggest global health threat of the 21st century [Reference Costello37]. There is much concern regarding the potential impact of global warming on infectious diseases [Reference McMichael, Woodruff and Hales38]. In a model constructed in Japan, the authors simulated the impact of global warming on the incidence of HFMD by varying vapour pressure, temperature, and relative humidity parameters and found that disease activity might increase by 7–14% [Reference Urashima, Shindo and Okabe31]. Based on projections by climate models, the Intergovernmental Panel on Climate Change in their Fourth Assessment Report indicated that the global average surface temperature would rise by 1·1°C to 6·4°C by the end of the 21st century [Reference Parry, Canziani, Palutikof and van der Linden39]. In a forecast conducted by the Hong Kong Observatory, it was estimated that by 2090–2099, in relation to 1961–1990, the annual mean temperature in Hong Kong would rise by 1·7°C to 5·6°C, with a combined mean of 3·5°C [Reference Leung, Wu and Yeung40]. These forecasts call for further attention on how HFMD trends may evolve in the coming decades. In fact, warmer winters in recent years might help to explain the higher observed winter peaks of HFMD detected by the sentinel surveillance system in Hong Kong. The present model demonstrates that using climate parameters helps to predict HFMD activity 2 weeks later. This can assist in better preparedness for the whole community prior to the actual upsurge of the disease.
ACKNOWLEDGEMENTS
We thank the Hong Kong Observatory for providing the meteorological data, all the doctors that participated in the sentinel surveillance system and Mr Simon Wong for providing the statistical advice for this study.
APPENDIX. Meteorological parameters used for examining the relationship with the weekly HFMD consultation rates
DECLARATION OF INTEREST
None.








