Article contents
The influence of air movement and atmospheric conditions on the heat loss from a cylindrical moist body
Published online by Cambridge University Press: 15 May 2009
Extract
It is emphasized that as heat exchange is controlled by the temperature of that boundary layer of molecular dimensions which separates a cooling body from its environment and from which evaporation occurs, attempts to relate heat loss with internal temperature have resulted only in empirical formulae. A rational formula involving the temperature of the evaporating surface is suggested, and it is shown how in the case of a system of sufficient simplicity all the terms can be either measured or derived from experiments.
The results of experiments with a small moistened cylinder are detailed illustrating the effect of wind velocity upon evaporative and convective heat loss under the one condition when the evaporating surface remains at constant temperature notwithstanding variations in wind, namely, when the whole system has been cooled to wet-bulb temperature. Evaporative loss is found to vary as V0.65, convective as V0.70.
Experiments are next described showing the effect of wind upon evaporative and convective losses when, the internal temperature being constant, the temperature of the evaporating surface fluctuates in consequence of varying wind velocity. Heat loss now varies very closely as V0.5 at velocities greater than 1 m./sec. At velocities below 1 m./sec. the same relation of heat loss to velocity obtains if due allowance be made for natural convection. This square root function is fortuitous, and heat loss varied between the square root and cube root of the velocity as the internal conductivity was diminished.
Attention is drawn to the impossibility of forming general conclusions from observations on any particular system, as the way in which the rate of heat loss varies with the velocity of the wind depends not only upon the internal conductivity of the system but also on its size and shape.
Observations are described showing the influence of varying the internal temperature on total and evaporative heat loss with constant wind velocity and constant atmospheric conditions. These experiments furnish data from which the surface temperature can be derived from measurements of evaporation, and show that the temperature of the surface and the rate of loss of heat by convection are both linear functions of the internal temperature at any one wind velocity. They also show that the values of the constants of the system derived from experiments at the temperature of the wet bulb are applicable when the cylinder is heated.
An analysis of the results of the experiments with varying internal temperature indicates that the temperature of the evaporating surface (ts) is related to the internal temperature (t1) and that of the wet bulb (tw) by the expression 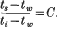 The value of C with varying wind velocity is ascertained by experiments, thus affording another means of arriving at the temperature of the evaporating layer. Values of ts obtained in this way are compared with those determined by observing the rate of evaporation and show reasonable agreement.
The value of C with varying wind velocity is ascertained by experiments, thus affording another means of arriving at the temperature of the evaporating layer. Values of ts obtained in this way are compared with those determined by observing the rate of evaporation and show reasonable agreement.
It is shown how, knowing the temperature of the evaporating layer, the constants of the system employed and the effect of velocity of wind upon heat exchange, the rate of loss of heat by evaporation and by convection under given conditions can be predicted. Instances of the agreement between predicted and observed values are given.
From the formula representing the influence of atmospheric conditions on heat loss it can be shown, by calculation, that if the wet-bulb temperature remains constant considerable variations in the temperature of the dry-bulb influence but slightly the heat loss from the moist cylinder.
It will be seen that the analysis of the effects of environmental changes on the heat loss from a simple physical system such as was used presents no serious difficulties. Such an analysis, unfortunately, does not enable deductions to be made with reference to systems of different physical characteristics. How observations on such systems can be related in other than a qualitative manner to the effects of corresponding changes on living creature differing in size and shape and degree of moistening of their surfaces is not clear. When account is taken of the ability of living beings to alter the vascularity of their surface tissues and so to vary the temperature of the body surface while other factors remain constant, the difficulties in relating the cooling of any physical system to the loss of heat from animals become painfully apparent.
The most hopeful method of assessing the effect of air movement and atmospheric conditions on the heat loss from the human body seems to be in terms of a subjectively determined standard such as the effective temperature scale of Houghton & Yaglou. The validity of such a scale has received support from observations by Houghton et al. (1924) and Vernon & Warner (1932) on the relation of pulse rate, body temperature, metabolism and other physiological variables to “effective temperature”.
- Type
- Research Article
- Information
- Copyright
- Copyright © Cambridge University Press 1939
References
REFERENCES
- 2
- Cited by


