INTRODUCTION
Parvovirus B19 (B19V), classified in the genus Erythrovirus, family Parvoviridae, infects only humans and replicates preferentially in erythrocyte precursors. In healthy, immunocompetent children, B19V causes erythema infectiosum or ‘fifth disease’, a benign febrile exanthematous illness [Reference Heegaard and Brown1, Reference Broliden, Tolfvenstam and Norbeck2]. High-titred viraemia occurs 1 week after infection, specific IgM becomes detectable by 12 days post-infection and IgG levels rise shortly after [Reference Anderson3, Reference Erdman4]. The development of the antibody response is linked to the clearance of the virus, although viral DNA has been detected in serum during convalescence [Reference Musiani5, Reference Lindblom6]. Infection in adults is associated with arthropathy in 20–80% of cases and both inflammatory arthritis and arthralgia may occur [Reference Woolf7–Reference Oiwa10].
Given the similarity in clinical presentations associated with the several viral infections that cause febrile exanthema (FE), differential diagnosis by laboratory testing is necessary, particularly in the context of epidemiological surveillance of measles and rubella [Reference Ramsay11–Reference Oliveira13]. In Argentina, the incorporation of the trivalent measles-mumps-rubella vaccine (MMR) as a national programme of obligatory but free vaccination starting in 1998, as well as massive immunization campaigns aimed at measles, rubella and congenital rubella syndrome (CRS) control, carried out between 2006 and 2009, led to a decline in cases of rash illnesses caused by these viruses. The last rubella case occurred in February 2009. On the other hand, although during last spring 17 cases were confirmed, measles had not been circulating since 2001 [14]. The decrease in measles and rubella cases focuses attention on erythema infectiosum; however, to date the only data gathered in Argentina on this disease include a school outbreak in Buenos Aires [Reference Alonso15] and the prevalence of specific IgG in pregnant women in Cordoba [Reference Pedranti16].
Due to the tropism of B19V for erythrocyte precursors, the infection can cause aplastic crisis in patients with previous haematological diseases, chronic anaemia (linked to persistent infection), in immunocompromised individuals, and hydrops and fetal loss in the congenital form of the disease [Reference Heegaard and Brown1, Reference Broliden, Tolfvenstam and Norbeck2]. For this reason, the characterization of the local epidemiology of B19V is important in the management of high-risk groups, such as pregnant women, patients with haematological diseases and immunosuppressed individuals who are seronegative for B19V.
Consequently, the goal of this study was to identify correlates of infection and immunity for B19V in FE cases in Cordoba, Argentina.
MATERIALS AND METHODS
Study population and clinical specimens
Serum samples collected in the context of the National Programme of Epidemiological Surveillance for the Control and Elimination of Measles, Rubella and CRS were used. According to this programme, which has nationwide coverage, cases of FE compatible with measles or rubella must be reported and diagnosed by laboratory testing. Cases suspected of measles are patients of any age presenting with fever of ⩾38°C and rash, plus one or more of the following: cough, rhinorrhoea, conjunctivitis; cases suspected of rubella are patients of any age presenting with fever and rash, plus one or more of the following: cervical, sub-occipital or retro-ocular adenopathy, arthritis, arthralgia. The study was performed with samples collected from January 2005 to December 2009 in the city of Cordoba, Argentina (where the reporting rate was around 50 cases per year). All available samples that were negative for measles- and rubella-specific IgM were included. We analysed samples from 141 patients with FE. The samples were taken at the onset of symptoms, when the suspected cases were reported. The age range was 2 months to 44 years [mean±standard deviation (s.d.) 8·99±9·56 years] and 62·4% of the patients were female. Samples from 2005 to 2007 were stored at −20° C and analysed retrospectively, while 2008 and 2009 sera were analysed prospectively as collected. A group of 31 healthy individuals covering a similar age range as the FE patients (8 months to 57 years, mean±s.d. 12·83±12·66 years) were enrolled as a control population; samples from the control group were collected with informed consent between 2008 and 2009. The study protocol was evaluated and approved by the Ethics Committee, Facultad de Ciencias Medicas, Universidad Nacional de Cordoba (Exp. no. 06-2008-46379).
Laboratory tests
Serology
Anti-B19V IgM and IgG were detected by commercial enzyme immunosorbent assays, specific for B19V major capsid protein VP2 (Biotrin, Ireland; 100% sensitivity and 100% specificity for both, IgM and IgG assays), following the manufacturer's instructions.
Nucleic acid extraction
Nucleic acids from each serum specimen were extracted using a procedure based on a method described by Boom et al. [Reference Boom17]. Briefly, a 50 μl-serum aliquot was incubated with 100 μl lysis buffer [0·1 m Tris (pH 6·4); 37 mm EDTA (pH 8·0); 0·22 g/ml Triton X-100; 1 g/ml guanidine isothiocyanate] and 10 μl silica for 10 min at room temperature. After spin down for 5 s the pellet was washed twice with 70% ethanol and once with acetone, dried at 56°C for 15 min, resuspended in 25 μl TE buffer (pH 8·0) and incubated for 15 min at 56°C. The silica was removed by centrifugation at 12 000 g and the supernatant was stored at −20°C until used in a PCR reaction.
PCR for the detection of B19V DNA
B19V DNA in serum samples was detected by nested PCR with primers targeting a region of the VP1/VP2 gene as published previously [Reference Cassinotti18, Reference Us19]. Each reaction contained 1 μl template in 10 μl with 0·02 U/μl Platinum Taq DNA polymerase in the buffer provided by the manufacturer (Invitrogen, USA), 0·2 μm of each primer, and 0·2 mm of each dNTP. The first round used 2 mm MgCl2, the primers 5′-AAGTACCCATATGTGTTA (nt 3775–3792) and 5′-GCATGAAGTTTTGGGGCT (nt 4154–4171), and a programme of 35 cycles as follows: 94°C (30 s), 45°C (30 s) and 72°C (1 min), with a final extension at 72°C for 10 min. In the second round MgCl2 was added at a 4 mm concentration and the primers were 5′-CAGAACTTCCTATTTGGGTA (nt 3818–3837) and 5′-CTGTACCTAAAAGCTGAAAA (nt 3956–3975); the cycling programme was similar to the first round except for annealing at 50°C (30 s). A standard B19V DNA (10 000 copies/ml), kindly provided by Dr G. Rodriguez (Laboratorio de Hemoderivados, Universidad Nacional de Cordoba) and a serum sample from a patient with erythema infectiosum confirmed by specific IgM and IgG detection were used as positive controls. Reagent controls and negative sample controls (nucleic acid extracted from negative serum samples) were also included. The sensitivity of the PCR assay described was estimated using serial dilutions of the B19V DNA standard; the assay had a limit of detection of 1000 DNA copies/ml. The final PCR product, with an expected length of 158 nt, was detected by 8·5% polyacrylamide gel electrophoresis and silver staining.
Analysis of clinical and epidemiological data
Clinical and epidemiological data associated with cases of primary infection by B19V were obtained from the official form submitted for notification of rubella/measles suspected cases. The χ2 test was applied for determination of statistical differences in the comparisons of prevalence, epidemiological trends and clinical manifestations, with a level of significance of 0·05.
RESULTS
Features of the study population
In the FE group, 118 (83·7%) of the 141 samples were collected between days 2 and 7 after the appearance of rash. The number of patients was equitable for each year of the period studied, but most of the reported cases corresponded to paediatric patients and occurred during winter and spring: 99 (70%) patients were in age groups 0 to <5 years and 5 to <10 years while 107 (75·9%) of the samples were collected during winter and spring. The cause of this bias is not clear, although it could be related to a prevailing notion of measles and rubella occurring mainly in children and with a marked seasonality of annual outbreaks (as in the pre-vaccination era) trending the notification of suspected cases. Based on information gathered during the notification process, clinical manifestations (other than exanthema and fever) that characterized the group of patients are indicated in Table 1.
Table 1. Clinical features in patients with febrile exanthema (FE) divided into patients with recent or active acute infection by parvovirus B19 (IgM+) and IgM− patients
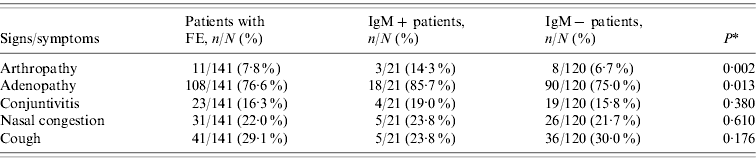
* Comparison between IgM+ and IgM− patients.
Markers of infection and immunity for B19V in patients with febrile exanthema and in the control group
The prevalence of viral DNA, specific IgM and IgG in the group of FE patients and controls are shown in Table 2. In the control group, although none had detectable specific IgM, three individuals had B19V DNA. The difference between DNA prevalence in the control group (9·7%) and FE patients (18·4%) was statistically significant (P=0·003), but the difference between IgG prevalence (41·9% vs. 51·1%) was not significant (P=0·064).
Table 2. Prevalence of markers of infection and immunity for parvovirus B19 (DNA, IgM, IgG), in the group of patients with febrile exanthema, 2005–2009, and in the control group
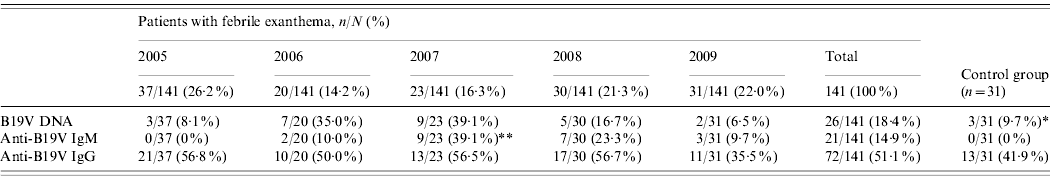
* Significantly different from the global prevalence of parvovirus B19 DNA in patients with febrile exanthema (P=0·003).
** Significantly different from other years (P<0·001).
The age range of patients positive for anti-B19V IgM (IgM+) was 2 months to 37 years. Only 10/21 (47·6%) IgM+ specimens had an associated positive determination of viral DNA (DNA+), while 19/21 (90·5%) of the IgM+ specimens were also positive for B19V-specific IgG (IgG+). On the other hand, B19V DNA was found in 16/53 (30·2%) of the specimens that were IgM−/IgG+ (immune patients). In these immune patients B19V DNA was significantly more frequently associated with children aged <15 years (P<0·001) and in male patients (P<0·001), as shown in Table 3.
Table 3. Prevalence of parvovirus B19 DNA in patients with febrile exanthema who were IgM−/IgG+

Values given are n/N (%).
Significant differences were found in the comparisons ‘<15 years old vs. ⩾15 years old’ (P<0·001) and ‘male vs. female’ (P<0·001).
Epidemiological aspects
Thirteen out of 21 (61·9%) patients with acute B19V infection (IgM+) were women, paralleling the 62·4% female patients in the FE group of the study population. In contrast, considering only the adult group (>20 years), all IgM+ patients were women.
The prevalence of specific antibodies and viral DNA by age group and season are shown in Table 4 and Figure 1. Since viral DNA can persist longer than anti-B19V IgM in a proportion of individuals (even immunocompetent ones), IgM+ was taken as the parameter indicative of recent or active acute infection. On this basis, teenagers (10–20 years) were the age group in which acute B19V infection was most prevalent (23·8%, Table 4), and the majority of the cases of acute infection occurred during winter and spring (Fig. 1). There is evidence to consider 2007 as an epidemic year (Table 2, Fig. 1). During this epidemic year, 8/9 cases (88·9%) occurred in children aged <5 years (P<0·001), uniformly distributed among the 0–4, 5–9, and 10–15 years age groups; moreover, 7/9 (77·8%) IgM+ cases presented during spring and the remaining 22·2% in winter, while 77·8% of the patients were female (P<0·001). In the post-epidemic year the frequency of B19V infection in adults was higher (almost double) than in previous years (data not shown).
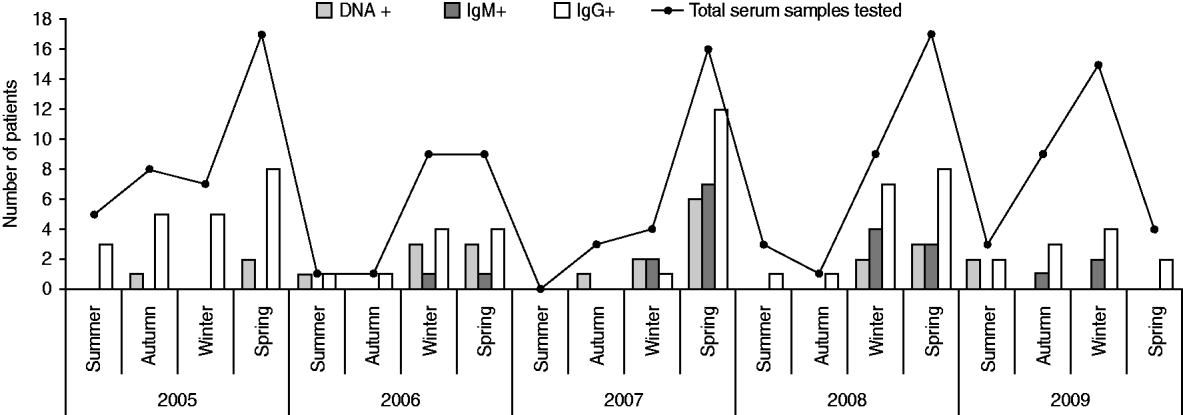
Fig. 1. Markers of infection and immunity for parvovirus B19 (DNA, IgM, IgG) by season and year, in patients with febrile exanthema.
Table 4. Markers of infection and immunity for parvovirus B19 (DNA, IgM, IgG) by age group in patients with febrile exanthema, 2005–2009
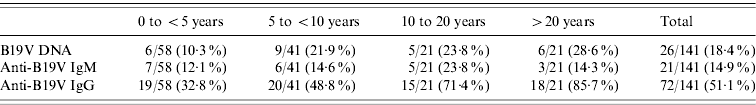
Values given are n/N (%).
Associated clinical aspects
Apart from the rash and fever (manifestations required to report suspected cases of measles or rubella), the most frequent clinical feature among B19V IgM+ patients was adenopathy, observed in 85·7% of the patients (Table 1). Only 14·3% of the B19V IgM+ patients reported arthropathy (arthritis/arthralgia), of which 100% occurred in women and 67% in the adult group (>20 years). The percentage of IgM+ patients with arthritis increased to 22·2% in 2007, the epidemic year. A comparison of clinical features between IgM+ and IgM− patients showed that patients with ongoing or recent acute B19V infection were more likely to present with arthropathy and adenopathy than patients with FE due to other aetiologies (Table 1). On the other hand, of the 16 patients in whom viral DNA was detected in the absence of specific IgM, nine (56·3%) had adenopathy and one (6·3%) reported arthropathy.
DISCUSSION
We communicate a follow-up study on the dynamics of B19V in Cordoba, Argentina, considering patients with FE compatible with measles and rubella over a 5-year period. Serological determinations confirmed that B19V was a significant local aetiological agent of this clinical entity, accounting for 14·9% of all the studied cases and 39·1% in the epidemic year (Table 2). The difference found in our population between the global percentage of IgM+ patients and the percentage of IgM+ patients in the epidemic year reflects the range of B19V-specific IgM detection in patients with rash diseases published in the region, which goes from as low as 3·3% up to 31·8%, with frequent recorded values of 12–13% [Reference Oliveira8, Reference Oliveira9, Reference Wermelinger12, Reference Oliveira13, Reference Mendonça20, Reference Larrañaga21].
Most acute infections were observed in teenagers (Table 4), but B19V contributes as the causative agent of FE in all age groups. Particular attention is drawn to recent or active acute B19V infection in childbearing age (teenagers and the >20 years group, Table 4). Due to the tropism of B19V for erythrocyte precursors, the infection can cause hydrops and fetal loss in the congenital form of the disease [Reference Heegaard and Brown1, Reference Broliden, Tolfvenstam and Norbeck2]. Therefore, primary B19V infection is an important factor to take into account during pregnancy, when there is a risk of vertical transmission of the virus to the developing foetus [Reference Enders22].
In regard to seasonality, cases of B19V acute infection in our study population occurred almost exclusively during winter and spring (Fig. 1). Similarly, other studies from different countries reported outbreaks of erythema infectiosum predominantly during late winter and spring [Reference Oliveira8, Reference Oiwa10, Reference Nicolay and Cotter23, Reference Goncalves and Dias24]. An epidemic outbreak occurred in 2007 (Table 2, Fig. 1), during which most cases of acute B19V infection were observed in children aged <15 years during spring, consistent with data published previously [Reference Oliveira8, Reference Oliveira13, Reference Mossong25]. Considering epidemics of B19V infection every 4–6 years [Reference Oliveira8, Reference Nicolay and Cotter23, Reference Mossong25], the next epidemic outbreak in our region can be expected in 2011–2013.
The global IgG seroprevalence (Table 2) was comparable to previously reported values in the general population, but when arranged by age groups (infants and pre-school children, school children, teenagers, adults, Table 4) it was higher [Reference Mossong25–Reference Röhrer29]. A study of patients with suspected B19V infection showed analogous results [Reference Wermelinger12]. As in that study, we focused on FE patients and B19V infections without fever or rash were not included. Highlighting a population of individuals with illness associated with B19V infection produced a higher prevalence than expected and thus the epidemiological data revealed might be biased by the study design.
There was no difference with respect to incidence of B19V infection between males and females when all cases of infection were considered. Conversely, significant differences between men and women were found in IgM+ cases in the adult group and during the epidemic year. Similar findings have been recognized by others [Reference Oliveira8, Reference Oiwa10, Reference Wermelinger12, Reference Nicolay and Cotter23]. These observations may have a direct relationship to the more apparent clinical presentation in women compared to men [Reference Woolf7, Reference Oliveira8], but this aspect could not be assessed in the present work, aimed at patients with clinical manifestations.
The detection of specific IgM was accompanied by the detection of viral DNA in 47·6% of the cases, which implies that by the time specific antibody levels were raised B19V DNA remained detectable in serum in about half of these inmmunocompetent individuals. When DNA+ patients were examined according to age range, the proportion of DNA+ individuals actually seemed to increase with age (Table 4); however, this picture may be influenced by the sample size in the age groups. In fact, B19V DNA has been found significantly more frequently in children than in adults with past infection (Table 3). The persistence of low viral DNA titres (in concentrations ~103 DNA copies/ml) in the serum of patients for years after infection has been reported [Reference Lindblom6, Reference Cassinotti and Siegl30], although the mechanism is not completely understood. In this work, B19V DNA was detected in 30·2% of immune FE patients (IgM−/IgG+). Adenopathy was associated with 56·3% of these, while one patient had arthropathy. Viral DNA was also detected in a proportion of the control group (individuals without symptoms) in the present study and in other studies [Reference Larrañaga21, Reference Söderlund-Venermo31, Reference Corcioli32]. Thus, although B19V DNA persistence is not necessarily associated with clinical manifestations, it is likely that there is a relationship between the persistence of B19V genome and concomitant virus replication and the generation of an inflammatory process in a proportion of such infected individuals [Reference Mitchell33–Reference Isa35]. Detection of the viral DNA in FE patients (DNA+/IgM+, 47·6%; DNA+/IgM−/IgG+, 30·2%) and in healthy individuals (DNA+/IgG+, 9·7%) may indicate that B19V can persist long-term in ~10% of immunocompetent individuals who experienced infection. We also found a significantly higher frequency of detection of B19V DNA in male than in female patients with past infection (IgM−/IgG+). The meaning of this association is still unclear, but could correlate to the fact that females make a stronger IgG response to B19V infection compared to males [Reference Vyse27], which has also been linked to more conspicuous clinical manifestations concurrent with immune complex formation (such as arthropathy) in women than men [Reference Woolf7].
B19V-related cases of FE in our population tended to be more associated with arthropathy and adenopathy than FE due to other causes (Table 1). Nevertheless, the comparison of clinical manifestations in IgM+ and IgM− cases showed a lack of differences in the medical presentations caused by different agents of exanthematous diseases, in agreement with previous reports [Reference Oliveira8, Reference Wermelinger12, Reference Mendonça20]. This highlights the value of B19V determination in the differential diagnosis of measles and rubella in FE patients, especially in the current context of the regional surveillance programme for measles and rubella elimination which is reaching its final phase (with a decrease in measles and rubella cases greater than 99%).
To conclude, this is the first follow-up study of markers of infection and immunity for B19V infection in Argentina. The results highlight the local impact of B19V as an aetiological agent of febrile exanthema and are noteworthy in local settings for medical management of patients in whom infection can pose a risk, i.e. immunocompromised individuals, pregnant women, and patients with haematological diseases susceptible to B19V.
ACKNOWLEDGEMENTS
This study was performed with grants from FONCYT-ANPCYT, Ministry of Science and Technology, Argentina (PICT 2007-00981) and SECYT-UNC (R-Secyt 214/2010). We are grateful to Dr Gonzalo Rodriguez (Laboratorio de Hemoderivados, Universidad Nacional de Cordoba) for kindly providing a B19V standard as a positive control for PCR and to Dr Teryl K. Frey (Georgia State University) for reviewing the manuscript.
DECLARATION OF INTEREST
None.







