INTRODUCTION
Pertussis (whooping cough) is an infectious respiratory disease caused by the bacterium Bordetella pertussis [Reference Hewlett, Mandell, Bennett and Dolin1, Reference Crowcroft and Pebody2]. It causes severe disease in infants and young children and prolonged cough in adolescents and adults [Reference Crowcroft and Pebody2, Reference Hewlett and Edwards3]. The disease can also lead to serious complications in adults with predisposing conditions, especially in the elderly [Reference Crowcroft and Pebody2, Reference Hewlett and Edwards3]. The immunization of infants and toddlers against pertussis, initiated worldwide in the early 1960s, reduced severe disease, complications and deaths in young children [Reference Cherry4–Reference Tan, Trindade and Skowronski6]. In previous decades, countries with a long history of vaccination reported increased pertussis rates in adolescents and adults [Reference Tan, Trindade and Skowronski6, Reference Skowronski7]. This can lead to increased exposure of young infants [Reference Wirsing von Konig5, Reference Wirsing von König8]. To some degree, this increase might also reflect the improved awareness and reporting by physicians or introduction of new diagnostic tests.
In 1960, Poland started mass vaccination with whole-cell component (DTwP) vaccine. Initially, a four-dose schedule was used (at 2, 3–4, 5–6, 18–24 months). The high vaccine coverage reduced reported pertussis rates, especially in the younger age groups [9]. In the mid-1990s, physicians reported more cases in all age groups [Reference Gzyl10]. Consequently, in 2004 Poland implemented an additional booster dose for young children at age 6 years with an acellular pertussis vaccine.
The aim of this study was to estimate pertussis incidence in patients with cough lasting >2 weeks presenting to their general practitioner (GP), and to compare the obtained estimates to the national reporting rates from the corresponding period.
METHODS
Between July 2009 and April 2011, we followed a cohort of patients registered to randomly selected GPs.
Study sites selection
We used a two-stage sampling procedure. First, we selected a random sample of 180 healthcare units, stratified by province and residence type (village, town <200 00 town 20-1 000 00 town >100 000 inhabitants), weighted on population in each stratum. We obtained the list of primary healthcare units from the register maintained at the Centre for Healthcare Information Systems (www.rejestrzoz.pl). The eligible units: (i) included at least one GP practice; (ii) had access to centrifuge and freezer; (iii) were able to identify persons registered to particular GPs; (iv) had permission of the unit director; (v) met the ethical requirements, and accepted compliance assurance procedures; (vi) had a minimum of 1000 patients registered at the practice. We selected up to three physicians in one healthcare unit, if they agreed to participate. We trained the GPs in the study procedures, and the nurses in specimen collection procedures.
Case definitions
We used a case definition compatible with the WHO pertussis definition (see Supplementary online material) [11]. We defined a suspected pertussis case as a person aged ⩾3 years, visiting his/her GP due to a persistent cough lasting ⩾14 days with at least one of the following symptoms: paroxysms of coughing, inspiratory whooping or post-tussive vomiting without other apparent cause, who gave informed consent for participation in the study. We defined a confirmed pertussis case as a suspect case, not vaccinated against pertussis in the previous 6 months, in which positive paired serology or detection of B. pertussis DNA by PCR from a nasopharyngeal swab confirmed recent pertussis infection.
Data collection
The GPs identified all suspected cases and listed them in a log forwarded each week to the study coordinators. The GPs interviewed each suspect case. The information on vaccination status was validated with official immunization records. The nurse collected a nasopharyngeal swab and a blood specimen. Thirty days following the initial visit, GPs scheduled a control visit, during which they re-evaluated the suspected cases and collected a second blood specimen.
The blood specimens were collected in EDTA-coated tubes. After centrifugation, plasma was placed in transport tubes. Plasma specimens and swabs were stored locally at –20°C and transported at 4-month intervals to the Department of Bacteriology, National Institute of Public Health (NIPH), serving as the National Reference Centre for pertussis diagnostics in Poland.
Serological investigations
Specimens were transported to the central laboratory at −70°C. We determined the concentration of IgG and IgA antibodies against a mixture of B. pertussis toxin (PT) and filamentous haemagglutinin (FHA) by a commercial enzyme-linked immunosorbent assay (ELISA) [NovaLisa Bordetella pertussis IgG (ref. BOPG0030) and NovaLisa Bordetella pertussis IgA (ref. BOPA0030); manufactured by NovaTec, ImmunDiagnostica, Germany] in accordance with the manufacturer's instructions. We diluted plasma specimens at 1:101. We tested cut-off calibrator and control specimens in duplicate. We divided the optical density (OD) of the specimen by the mean OD of the cut-off calibrator, and multiplied by 10. We adjusted the cut-off levels for the classification of pertussis seropositivity based on a validation sub-study of 160 age-stratified specimens collected from healthy individuals, as published elsewhere [Reference Rastawicki12]. We considered as positive the ELISA result when IgA and/or IgG antibody concentration exceeded by 3 s.d. the arithmetic mean of the respective antibody titre in the above-mentioned healthy individuals. To improve the reliability of serological testing, we detected antibodies in two classes: IgA as an indicator of recent infection, especially in adults; and IgG as indicator of immune status due its long persistence in blood. We carefully interpreted the antibody response in recently immunized patients. The high threshold for antibody titre was chosen due to the possibility of unspecific reactions, as well as individual differences in IgA and IgG response following infection and vaccination.
Molecular investigations
We isolated B. pertussis DNA from the nasopharyngeal swabs using a High Pure PCR Template Preparation kit (Roche, Germany) according to the manufacturer's protocol. We detected DNA using real-time PCR as described by Kösters et al. [Reference Kösters, Riffelmann and Wirsing von König13]. We used a Rotor-Gene Probe PCR kit (Qiagen, Germany) to prepare the PCR reaction mix, and the Rotor-Gene instrument (Qiagen) for the PCR reaction.
Denominator populations
To obtain the denominators for the incidence rates, we aggregated the number of persons registered in participating practices by sex and age group (status on 31 December 2009). We assigned to each member of the population 1 person-month of exposure for every month of practice participation. For the national reported pertussis rates, we used Poland's 2010 mid-year population estimates [14].
Data analysis
We calculated pertussis incidence rates in the studied population. For the numerator, we used laboratory-confirmed cases of pertussis. We compared the age-specific pertussis rates estimated in our study population with the annual rates of pertussis cases aged ⩾3 years reported to the Polish national surveillance from July 2009 to April 2011.
Statistical adjustments
Because not all eligible patients who met the inclusion criteria were tested, we first corrected the observed pertussis case counts for under-ascertainment. To do this we estimated the probability of testing in eligible patients with a logistic regression model. The model included fixed covariates for age, sex, region, and urbanization category and a random intercept for each GP. Cases were categorized by these factors, and the adjusted number of cases in each category was estimated by summing the reciprocals of the estimated probabilities from the model. For this analysis, we used the ‘xtmelogit’ command in Stata [15].
To adjust our estimates to Poland's population structure and the two-stage sampling scheme, we used the following weighting procedure. First, we computed the sampling weights for each region by multiplying the reciprocals of the proportion of medical units recruited and the proportion of GPs participating in each unit. These sampling weights were then ‘raked’ so that weighted totals would match census totals for regions, urbanization categories, and age by sex categories [Reference Lohr16]. For this analysis we used the ‘survwgt’ command in Stata [Reference Winter17].
To get the final esimate of pertussis incidence (events/100 000 person-years), we employed survey-weighted Poisson regression of the weighted case counts. Standard errors and confidence intervals were based on variation between the 34 medical units, which were the first-stage clusters in the design.
Protection of human subjects
GPs provided detailed information about the study to all eligible subjects and obtained consent from the participants. For patients aged ⩽18 years, GPs obtained informed consent from their legal guardians. The hard copies of the consent forms were stored in GP practices with the medical documentation. Referring physicians were informed of laboratory investigation results. The materials sent to the laboratory and the investigation forms sent to the coordination centre were de-identified to maintain the patients' privacy. The study protocol was approved by the Ethical Committee of the National Institute of Public Health – National Institute of Hygiene.
RESULTS
Study site selection
We selected 34 of 180 sampled health units (Fig. 1) and recruited 78 general practitioners in the selected units. We followed the cohort of 158 863 persons for variable periods, starting at the first recruited practice on 20 July 2009 and ending with the last unit on 30 April 2011. The follow-up period was 197 955 person-years. Compared to the 2009 census data, the study population reflected well the Polish population in terms of age and sex distribution, but underrepresented inhabitants of medium-sized towns (Table 1).
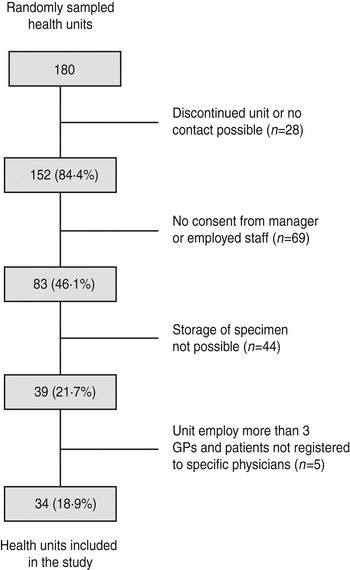
Fig. 1. Sampling of the study sites, Polish Pertussis Study, July 2009–April 2011.
Table 1. Studied population in relation to the general population of Poland, by age group, sex and urbanization degree, July 2009–April 2011

Pertussis case ascertainment
During the observation time, 3864 patients with cough consulted the participating physicians (1·95 visits/100 person-years). Of those, 1852 (48%) were suspect cases. Of those, 1232 (67%) were recruited and tested (1144 by both PCR and serology, 79 only by serology and nine only by PCR (Fig. 2)). Patients were less frequently recruited and tested in the youngest age groups and in inhabitants of medium-sized towns (Table 2). Of the 1232 patients recruited, 993 attended the control visit at 30–60 days. We confirmed 288 pertussis cases (278 only by serology, eight only by PCR, and two by both serology and PCR). Of 1232 patients recruited, 344 (28%) were vaccinated against pertussis according to GP documentation and 231 (19%) were current smokers. Compared to patients testing negative, pertussis cases had similar demographic and clinical characteristics, but were less frequently vaccinated and more commonly current smokers (Table 3). Pertussis cases confirmed by PCR had shorter average duration of cough than those confirmed by PCR (Table 3).
Table 2. Comparison of patients recruited to the study with patients who declined to participate, by age group, sex and urbanization degree, Poland, July 2009–April 2011

Table 3. Selected demographic and clinical characteristics of tested patients, by laboratory confirmation status, Poland, July 2009–April 2011

Pertussis incidence
The crude incidence rate was 145·5/100 000 person-years (Table 2). The highest rates were in patients aged 55–59 years (244·8), and lowest in those aged 3–5 years (43·0). Out of 288 pertussis cases, 11 (4%) were referred to hospital and seven (2%) were reported to surveillance.
The adjusted incidence rate was 201·1/100 000 person-years [95% confidence interval (CI) 133·9–302·0]. The highest adjusted rates were in the 15–19 years group (456·5, 95% CI 239·3–870·8), and the lowest in the 25–29 years group (94·0) (Table 4). The highest incidence was in females compared to males (218·5 vs. 180·5, respectively), and in inhabitants of large towns compared to inhabitants of medium towns (237·2 vs. 173·8, respectively).
Table 4. Univariable and multivariable analysis of pertussis testing results in the studied cohort, Poland, July 2009–April 2011

IR, Incidence rate; CI, confidence interval; rates calculated/100 000 person-years.
Extrapolating the obtained results to the Polish population aged >3 years, we estimated 74 319 pertussis cases (201·1 cases/100 000*36 954 352). Considering the confidence levels, the estimated number of pertussis cases could be as low as 49 497 and as high as 111 598.
Ratios to national surveillance
During the study period, Polish physicians reported 2254 pertussis cases aged >3 years (annual reported rate of 3·3/100 000 inhabitants). The highest reported rates were in those aged 3–5 years (35·8) and 10–14 years (28·8). Hospital physicians reported 897 cases.

Fig. 2. Recruitment of study participants, Polish Pertussis Study, July 2009–April 2011.
Comparing the adjusted pertussis rate with the reported incidence, we obtained an overall reporting ratio of 61. The age-specific reporting ratios ranged from 4 in those aged 3–5 years to 167 in those aged 65–69 years (Fig. 3).

Fig. 3. Comparison of age-specific pertussis incidence estimated in the study population (black line) with reported incidence (grey line), with reporting ratios displayed for each age group, Poland, July 2009–April 2011.
DISCUSSION
Summary of key findings
This is one of the largest population-based studies of pertussis to date, comprising almost 200 000 person-years of observation in primary care. Because of random sampling of the practice populations forming the observed cohort, we were able to follow a sample of Poland's population representative in terms of age, sex, region of residence and urbanization level. We estimated an annual incidence of 201·1 pertussis cases/100 000 person-years. Based on extrapolation of the present study results to the entire Polish population, we estimated the annual number of 74 319 patients with pertussis consulting GPs which is 61 times higher than the annual number of 1228 cases reported to surveillance in the corresponding period. The reporting ratio ranged from 4 in those aged 3–5 years, to 167 in persons aged 65–59 years.
Comparison with previous studies
Our estimates should be compared with caution to previous studies estimating the incidence of symptomatic pertussis in general population [Reference Schmitt-Grohé18–Reference Lasserre23]. Most of the above-mentioned papers had a sampling design that would exclude the external validity of obtained results. The sample sizes of the above-mentioned studies were limited, recruiting from 153 [Reference Nennig19] to 356 [Reference Miller20] patients with cough. The observed population samples consisted of either a sentinel network of GPs [Reference Gilberg22, Reference Lasserre23], patients of 10 clinics [Reference Strebel21], patients referred to one GP practice [Reference Miller20], a convenience sample consisting of members of a health plan [Reference Nennig19], or household contacts of vaccine recipients [Reference Schmitt-Grohé18]. Based on the above-mentioned studies, the incidence of symptomatic pertussis requiring medical attention ranged from 66 [Reference Gilberg22] to 508 [Reference Lasserre23] cases/100 000 person-years. Our estimate is in the same range; however, it had a lower uncertainty range due to the larger sample size and screening of a wide range of age groups.
Only one study performed on 212 persons aged 10–49 years living in Minnesota permitted estimation of age-specific incidence rates of symptomatic pertussis, establishing the highest rate in those aged 10–19 years (997 cases/100 000 person-years) [Reference Strebel21]. This was in agreement with the European Sero-Epidemiology Network (ESEN) study, which ascertained higher titres of anti-PT antibodies indicative of recent infection in children and adolescents than adults [Reference Pebody24, Reference Kretzschmar, Teunis and Pebody25].
We compared the pertussis estimates to the routine surveillance data. We observed important under-reporting, especially in persons aged ⩾55 years. Lower reporting ratios in children may indicate much higher awareness of pertussis by patients and physicians in younger age groups. As in other countries [Reference Hewlett and Edwards3, Reference Skowronski7, Reference Lasserre23], Polish physicians may still consider pertussis as a disease of childhood and rely on typical symptoms when referring for testing. Since the cost of the laboratory testing has to be covered by the reporting physician, GPs are commonly not referring suspect pertussis cases for testing, especially uncomplicated adult cases. With such low surveillance sensitivity, it will be difficult to adapt the immunization schedule to changing pertussis epidemiology, or track potential reasons for waning immunity [Reference Mooi, van der Maas and De Melker26].
Study limitations
The case definition criteria allowed only recruitment of patients with cough lasting >14 days. This criterion prevented efficient use of PCR for pertussis diagnosis, as this method is most reliable in the first 3 weeks of cough [Reference André27, Reference Guiso28]. We could miss patients with the most severe symptoms and rapid progression, especially in small children and persons with chronic diseases, who would consult their physician within the first 2 weeks of cough.
Potential loss to follow-up could result from unmonitored changes in the study population, e.g. moving some patients to older age groups during the study period. Moreover, physicians registered new patients and unregistered others, e.g. those who moved away or died. We could not assure a detailed monitoring of changes in the study population as some practices maintained paper registers of patients. Since the median duration of follow-up was 14 months, we assumed that changes in the population structure were balanced and would not lead to important biases of our estimates. Loss to follow-up could also occur if patients did not consult the GP practice, for example visiting another GP while on holiday or going directly to a hospital emergency department. However, due to the organization of healthcare in Poland, where patients are linked with one practice in relation to all basic services, we assumed that GPs would be informed about any important health events, and would include them in the screening logs. We addressed the potential loss to follow-up by thorough training and weekly monitoring of screening logs maintained in each practice. Differences in study site performance were addressed by monitoring visits of the lowest and highest performing sites throughout the study period, during which time we reviewed with local investigators the screening log procedures and inclusion criteria. Additionally, the multivariable analysis allowed adjustment of results for under-ascertainment of patients meeting the inclusion criteria.
We used a commercial ELISA for diagnosis of pertussis cases. As documented in a comparative study, ELISA tests have limited reliability, and do not allow comparisons of results obtained in different populations, as they often give positive results in healthy subjects [Reference Riffelmann29]. We decided to use the laboratory method applied routinely in surveillance because our primary goal was to estimate reporting ratios for Poland. To avoid false-positive results, we adjusted the cut-off values for particular classes of antibodies to the Polish population background antibody concentrations [Reference Rastawicki12]. Since surveillance reports are often based on single serological tests and commercial cut-off values, false-positive reporting could result in underestimation of our reporting ratios. Moreover, our estimates and surveillance figures cannot be directly compared to pertussis rates reported in other countries, which use heterogeneous microbiological methods for pertussis confirmation [Reference He30].
CONCLUSIONS
Despite the important limitations discussed above, our study enabled the establishment of precise age-specific estimates of pertussis incidence in the general population of Poland. Due to the unknown proportion of adults visiting their GPs with symptoms of cough, these estimates should be considered as minimal pertussis rates. The results of our study point to the unidentified burden of pertussis in adults and highlight the need to improve current surveillance systems and clarify the role of different adult age groups in pertussis transmission to children, as this could have important implications for vaccination policies.
SUPPLEMENTARY MATERIAL
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/S0950268813001684.
ACKNOWLEDGEMENTS
We thank all participants, GPs, nurses, laboratory and administrative staff who took part in the study. We thank Maciej Polak for his creative assistance in study coordination. This work was supported by an unrestricted research grant from GSK Biologicals. The sponsor did not have any role in planning, implementation, data analysis or preparation of the manuscript.
DECLARATION OF INTEREST
H.C. has received travel grants from Sanofi Pasteur, GlaxoSmithKline and Baxter, and remuneration for the conduct of clinical trials from GlaxoSmithKline, Pfizer, Sanofi Pasteur and Baxter. I.A.P-S. has received travel grant from GlaxoSmithKline. E.K. has received honorarium from GlaxoSmithKline.









