INTRODUCTION
Vaccination has a central role in the control of pertussis. For example, in the USA, widespread use of pertussis vaccine is responsible for more than a 100-fold decline in its prevalence [Reference Long, Edwards, Long, Pickering and Prober1].
Surveillance data are essential for developing and evolving strategies against pertussis. In Japan, as in other countries, national surveillance data are available from a passive surveillance system [2, Reference Tan, Trindade and Skowronski3]; Figure 1 shows the general epidemiology of pertussis in Japan, reported from 3000 paediatric clinics [4, 5]. The data illustrate that, although incidence of pertussis in infants has been stable, its incidence in other groups has been increasing in recent years. This increasing trend of pertussis is similar to observations in other countries, which is possibly attributable to waning vaccine-induced immunity with advanced age and heighted awareness [Reference Halperin6]. However, this type of data is of limited value because of possible underreporting [Reference Edwards7] and a lack of clinical information such as disease severity or outcomes of patients.
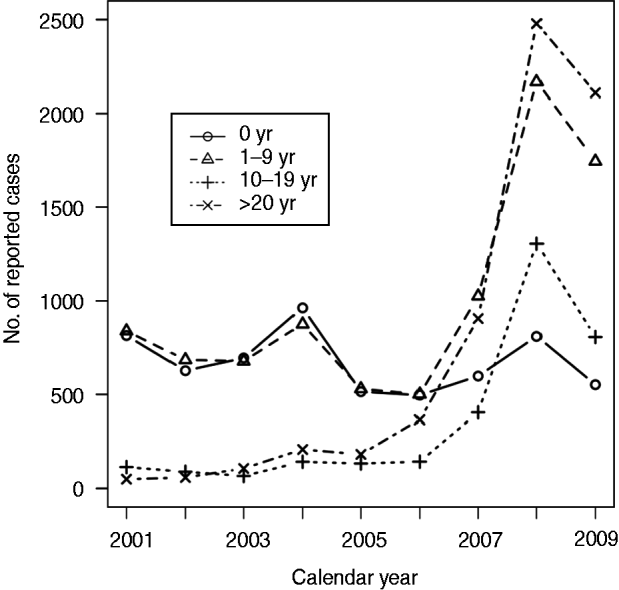
Fig. 1. Reported incidence of pertussis cases in all age groups in Japan; data were reproduced from references 4 and 5.
The aim of this study was to describe the epidemiology of pertussis among Japanese infants by utilizing a Japanese national hospital discharge database, which is less vulnerable to surveillance artifacts [Reference Cortese8, Reference Murphy9]. To the best of our knowledge, this is the first study to report the incidence of nationwide hospitalizations due to childhood pertussis in Japan.
METHODS
Approval for this study was obtained from the Institutional Review Board of the University of Occupational and Environmental Health Japan. This retrospective cohort study used data from the Diagnosis Procedure Combination (DPC) database of Japan.
Data source
The DPC database is an all-payer inpatient care database in Japan containing administrative claims data [Reference Fushimi10, Reference Horiguchi11]. Inpatients are assigned, upon discharge, to one of 2347 DPC groups (17 major diagnostic categories, 520 diseases) by combining three elements: diagnoses coded with the International Classification of Diseases and Related Health Problems, 10th Revision (ICD-10) codes; surgical procedures coded with Japanese K-codes; and comorbidities at admission and complications after admission coded with ICD-10 codes. Trained staff in each participating hospital accurately record the dates of all major and minor procedures and of drug administration and device use and therefore submissions of procedure codes are believed to be complete.
The DPC database contains various clinical and administrative data including diagnoses and procedures, drugs, devices, and length of stay (LOS), discharge status including in-hospital death, and costs. It does not contain patients' medical history including vaccinations, signs and symptoms, and laboratory data.
The number of participating hospitals was 262 in 2006, 926 in 2007, and 855 in 2008. Data are collected over 6 months (between 1 July and 31 December) each year, and are maintained by the DPC Research Team. Since 2008, data from about 2·86 million inpatients have been compiled, representing about 40% of all acute-care inpatient hospitalizations in Japan.
Case identification and definition of complications
Pertussis-specific ICD-10 codes of A370 and A379 were used to capture the eligible cases aged <12 months. The age in months at hospital admission was calculated from the age in days at admission divided by 30·4375 [4]. We also examined possible underlying conditions in hospitalized infants. Based on a previous study, we defined pertussis-related complications [Reference Cortese8]. Procedure codes were also examined for expected procedures (e.g. mechanical ventilation and extracorporeal membrane oxygenation).
We also investigated the incidence of pertussis-associated hospitalizations in children aged 1–3 years for comparison with infants.
Vaccination schedule during infancy in Japan
Table 1 summarizes the routine immunization schedule during infancy in Japan; each vaccine should be given separately according to law during the set period. With respect to pertussis vaccine, the first dose of pertussis vaccination combined with diphtheria and tetanus toxoids (DTP) is recommended for infants at age 3–6 months; the pertussis vaccine has been acellar type since 1981. The subsequent two doses are then given at intervals of 3–8 weeks, up to age 12 months. Bacillus Calmette–Guérin vaccine can be given from birth; however, a minimum age of 3 months is recommended to ensure that the infants do not have a contraindication to a live vaccine (e.g. severe combined immunodeficiency). Therefore, infants living in Japan typically receive their first vaccine at the age of 3 months or even later. The vaccination schedule in each infant is on a voluntary basis; the timing of the sequence of vaccination depends on multiple factors involving the opinions of healthcare providers and parental preference [Reference Kato12].
Table 1. Routine vaccination schedule among Japanese infants, 2006–2008Footnote *
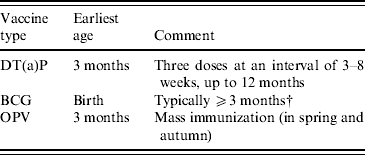
DT(a)P, Diphtheria-tetanus-(acellular)-pertussis vaccine; BCG, Bacillus Calmette–Guérin vaccine; OPV, oral poliovaccine.
* Each vaccination given separately.
† Based on the recommendation of experts: see details in Methods section.
Vaccination coverage of the three doses of DTP is reported as 98·7% in Japan; however, nationwide age-specific coverage data are limited [13].
Data analysis
SPSS version 17.0 (SPSS Inc., USA) was used for all statistical analyses. To describe continuous data, we presented data as mean ± standard deviation or median plus interquartile range (IQR) where appropriate. We used the Student t test to compare parameters between two groups; for comparisons among three groups, analysis of variance was used; a P value <0·05 was considered statistically significant.
RESULTS
Demographic data of patients
A total of 660 pertussis patients were identified (Fig. 2). The incidence peaked at age 1 month, with a median age of 4 months (IQR 2–6 months). The incidence decreased gradually from ages 1 to 3 months and a sharp decline was observed at age 3 months when the earliest DTP was possible in Japan (Fig. 2); the incidence continued to decrease up to 9–10 months of age. Of the 660 patients, 366 (55·5%) were old enough (⩾3 months) to be eligible to have received at least one dose of DTP vaccine. During the study period, there were 207 000 admissions of infants for all causes; thus, pertussis accounted for 0·32% of infant hospitalizations. During the same period, the number of pertussis hospitalizations for children aged 1–3 years was 141 (n=107, 22 and 12 in children aged 1, 2 and 3 years, respectively); thus, infants were at the highest risk of hospitalization due to childhood pertussis.
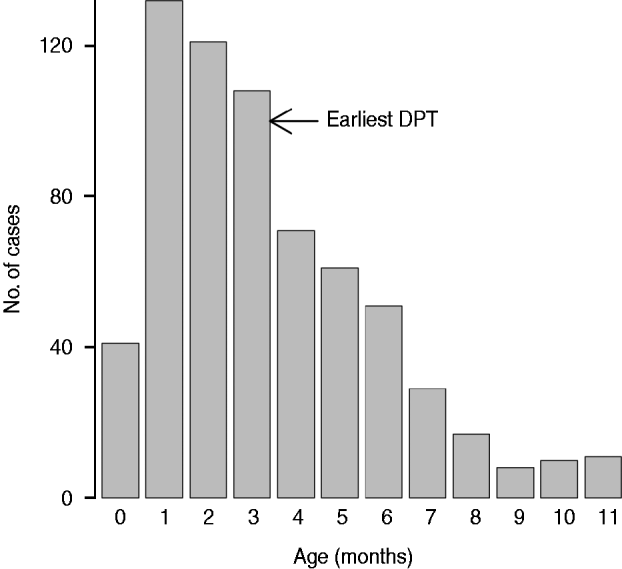
Fig. 2. Age distribution of 660 infants hospitalized for pertussis.
Pertussis affected both sexes equally (male 348, female 312). Chronic medical conditions were found in three (0·45%) cases, each of whom had congenital heart disease. Thus, most of the hospitalized infants were otherwise healthy.
Hospital course
Patients were discharged in a median of 8 days (IQR 6–11 days). LOS was age-dependent and shorter with older age (mean 10·2, 8·9 and 8·8 days in infants aged <3 months, 3–5 months and 6–11 months, respectively, P=0·014).
In total, 228 (34·5%) patients were treated with erythromycin, 324 (49·1%) with clarithromycin and 123 (18·6%) with other antibiotics (e.g. minomycin, azithromycin). LOS did not differ between patients treated with erythromycin and those treated with clarithromycin (mean 9·5 vs. 9·8 days, P=0·68).
Complications
We found 162 (24·5%) cases with complications (Table 2). These included respiratory, neurological and other complications. The prevalence of complications was highest in infants aged <3 months (68/294, 23·1%); however, complications were not uncommon in older infants who were eligible for DTP vaccination (15·0% in infants aged 3–5 months and 22·2% in infants aged 6–11 months).
Table 2. Proportion of hospitalized infants with specific complications according to age group
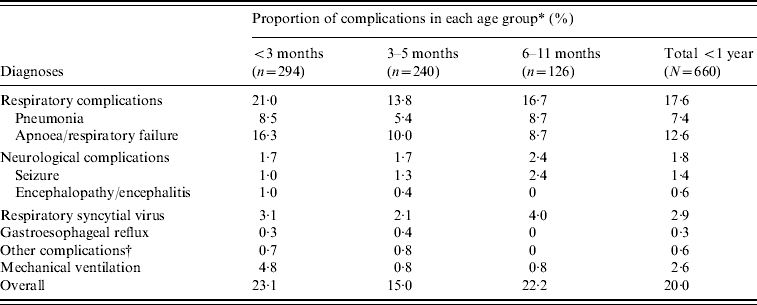
* One patient could have >1 complication.
† Includes heart failure (n=3) and haemorrhage (n=1).
Mechanical ventilation was required for 17 (2·6%) patients. Of these patients, infants too young to have been vaccinated for pertussis accounted for 14 (82·4%) cases; however, three infants were old enough to have been eligible to receive the DTP vaccine (age 4 months, n=2; age 6 months, n=1).
Among the cases recorded, we found a 1-month-old infant who suffered a fatal outcome, despite the use of extracorporeal membrane oxygenation. An autopsy was not performed.
DISCUSSION
We present infantile epidemiology of pertussis using an administrative database; our report is the first to describe the incidence of nationwide hospitalizations attributed to pertussis in Japanese infants.
Surveillance data obtained from a passive notification system often do not reflect the true burden of pertussis because of incomplete reporting and difficulties in diagnosis [Reference Tan, Trindade and Skowronski3]. For example, a survey in the USA illustrated that a significant amount of underreporting was likely in 5–6 times more cases than was reported to the Centers for Disease Control and Prevention [Reference Edwards7]. A hospital discharge database is less sensitive to surveillance artifacts, such as underdiagnosis and underreporting, and is a better data source for the determination of the epidemiology of pertussis [Reference Cortese8, Reference Murphy9]. Therefore, we conducted this survey using the Japanese administrative DPC database, focusing on infants who were at high risk of pertussis-related hospitalizations, complications and deaths.
Our study highlights the following issues in Japan. First, infants old enough to be eligible for at least one dose of DTP vaccine accounted for more than half of infant hospitalizations attributed to pertussis. Second, pertussis-related complications were not uncommon in infants eligible to receive the DTP vaccine. These results were in contrast to data in countries where vaccination coverage was high and timely. For example, in the USA and Australia, hospitalizations and complications attributed to pertussis were infrequent among infants old enough to be eligible for the pertussis vaccine [Reference Cortese8, Reference Wood14]. However, in Germany, where delayed DTP vaccination is common, there was a high burden of pertussis [Reference Hellenbrand15], which was similar to the findings from our data.
We postulate that the delay in receiving the first dose of DTP vaccine was responsible for this morbidity for two reasons. First, although data on vaccination coverage are scarce in Japan as described above, a survey in one medium-sized city showed that the median age of the first DTP vaccination was 7 months as presented in Figure 3 [Reference Kato12]. Second, previous studies have indicated that even a single dose of DTP vaccine is highly efficacious in preventing hospitalizations and complications due to pertussis [Reference Greenberg, von Konig and Heininger16, Reference Juretzko17]. Based on these evidences, we suggest that a delay in DTP vaccination is the most likely explanation for the morbidity found in our study.
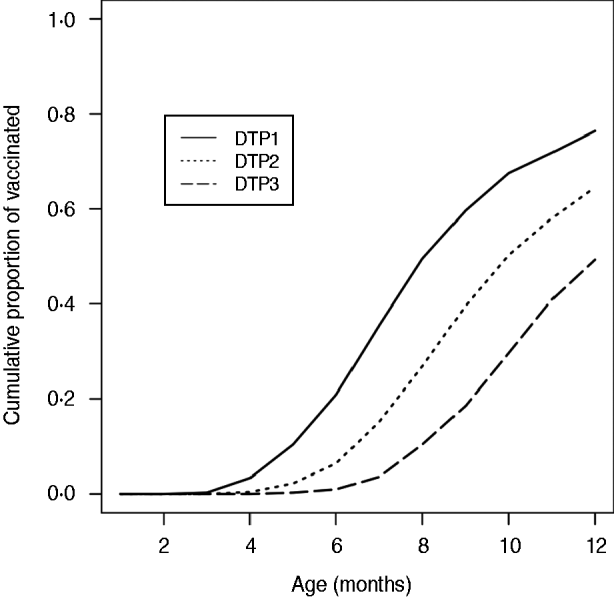
Fig. 3. Infant pertussis vaccine coverage according to age group in Gamagori, a medium-sized city in Aichi, Japan. (Data obtained from Kato et al. [Reference Kato12], courtesy of Dr Masahiro Kato.)
Vaccination failure or changes in the organism could be another explanation for the many cases of morbidity among vaccine-eligible infants, as has been reported in other countries [Reference Ntezayabo18, Reference Mooi, van Loo and King19]. However, neither possibility has been reported in Japan. A recent survey, for example, showed that the pertussis strain circulating in Japan remained unchanged [Reference Kikuchi20]. Moreover, another recent study suggested that current vaccination in Japan effectively prevented pertussis [Reference Okada21]. Therefore, we believe that there is no evidence to show reduced efficacy of vaccines against circulating strains in Japan.
In our study, infants aged 0 month were hospitalized less frequently than infants of older age groups. There are several explanations for low incidence of hospitalizations among infants aged 0 month. For example, maternal antibody may have a role for protecting infants from pertussis. Alternatively, a long incubation period (5–21 days) may explain low incidence of hospitalization in the youngest infants: infants only exposed at age ⩽2 weeks would develop pertussis symptoms at age 0 month.
Several limitations should be acknowledged. Limitations of this study include those intrinsic to a large database analysis. There may be variations in the diagnostic criteria between hospitals. Similarly, the accuracy of ICD-10 coding for pertussis is not established [Reference Cortese8]. The coverage rate of the DPC database and data collection period could be other limitations. The database represents only 40% of hospitalizations in Japan and this limitation makes comparison of the incidence of pertussis hospitalization with other countries difficult. Similarly, the data were collected for 6 months and pertussis epidemics may have seasonal variety, which might influence the number of cases. However, surveillance data including all age groups suggest that there is no or minimal seasonal variation of pertussis in Japan [22]. Therefore, we believe that the number of cases during the unobserved period (from January to June) was similar to that of the study period and that our data represent the burden of pertussis-related hospitalizations in Japanese infants.
In summary, we presented the descriptive epidemiology of infant hospitalizations for pertussis in Japan. Our data suggest that the vaccination schedule against pertussis in Japan is neither age-appropriate nor timely. The Japanese Pediatric Society recently published a recommended immunization schedule, in which the Society recommends that DTP should be given as early as age 3 months [23]. We believe that adherence to this schedule is the key to enhancing a timely vaccination schedule including pertussis vaccination.
ACKNOWLEDGEMENTS
This study was funded by Grants-in-Aid for Research on Policy Planning and Evaluation from the Ministry of Health, Labour and Welfare, Japan. The authors thank Dr Masahiro Kato for providing age-specific vaccine coverage data for the city of Gamagori.
DECLARATION OF INTEREST
None.







