INTRODUCTION
Rotavirus is the most frequently identified agent of infectious acute gastroenteritis (AGE) in unvaccinated children under 5 years of age [1–Reference Redondo, Cano and Simón4]. It is associated with significant morbidity and cost across Western Europe [Reference Le Saux5, Reference Ogilvie6]. In Europe as a whole, the median length of stay (LOS) for rotavirus hospitalizations is 4·8 days (range 2–9·5) [1, Reference Wildi-Runge7], 1·4 days longer than the average stay in North America [Reference Mast8]. Moreover, hospitalization charges for rotavirus among European children ranges from €1417·44 to 2263·79 per admission [Reference Marsella9, Reference Giaquinto10]. For example, the average LOS in Spain is 4·7 days [standard deviation (s.d.) 4·9 days], at an average cost of €1580·33 per admission, which translates to approximately €2·86 million in annual direct costs that are paid out by the Spanish National Health System (SNHS) and another €50 million in estimated societal costs [Reference Diez-Domingo11]. Compared with healthy children, vulnerable children with co-morbidities have, on average, 8 days greater LOS and six times higher hospital costs (P < 0·001) [Reference Pockett12]. Children aged 6 months and younger are also at increased risk of prolonged LOS from rotavirus [Reference Rowinsky13].
After implementing a rotavirus vaccination program during the period between 2008 and 2009, the reduction in costs associated with hospitalizations in the USA reached US$ 242 million [Reference Desai14, Reference Kilgore15]. Likewise, subsidized vaccination programs in Europe led to an annual decrease of 74% in the incidence rate of rotavirus hospitalizations after such period, representing a 73% annual reduction of direct costs [Reference Paulke-Korinek16, Reference Zlamy17]. Mean gross cost savings after implementation of rotavirus vaccination are expected to be €304 per avoided case; and up to 59% of total savings would be due to herd protection [Reference Karmann, Jurack and Lukas18]. In Spain, the reduction of the incidence of rotavirus hospitalizations during 2008–2009 was 33·1% [Reference Gil-Prieto19], similar to the 25–36% decrease observed in other European regions with low-to-moderate commercialization [Reference Dudareva-Vizule20]. Moreover, nosocomial acute rotavirus gastroenteritis (ARGE) decreased by 37·1% after the period 2007–2009 [Reference Redondo-González21]. This is consistent with a low-to-moderate rotavirus vaccine coverage in Spain ranging from 17% in 2007 to 38% in 2009 [Reference Redondo, Cano and Simón4].
Diagnosis-related groups (DRGs) are indicators of hospital resource consumption and performance. Hospitalized cases classified as belonging to a particular DRG are characterized by a homogenous resource consumption pattern, which involves both direct and indirect hospital costs. Thus, cases within the same DRG are medically and economically similar. DRGs use was approved by the SNHS in 1997 [Reference Rivero22]. Still, scientific publications on costs analyses of DRGs in infectious diseases in Europe are scarce despite their growing importance for determining hospital reimbursement [Reference Gómez23–Reference Mannocci28]. Specifically, a national estimate of reduction in costs following the decrease in rotavirus hospitalizations [Reference Redondo, Cano and Simón4, Reference Gil-Prieto19] has not been estimated to date.
Our aim was to quantify the impact on hospital LOS and costs after the longest time period in which both Rotarix® and Rotateq® were commercially available in Spain.
METHODS
Design, setting, and data source
This is a retrospective cohort study of community-acquired ARGE requiring hospital admission in Castile-La Mancha (CLM), Spain, from 1 January 2003 to 31 December 2009. CLM is a region with a population of over 2 million inhabitants and 19 tertiary referral hospitals. CLM have five major cities (provinces): Guadalajara, Toledo, Ciudad Real, Cuenca, and Albacete. Patients with ARGEs admitted to one privately owned hospital and 12 referral hospitals belonging to the Health Service of Castile-La Mancha (SESCAM), were identified from the Minimum Basic Data Set (MBDS), which uses the codes set forth in the International Classification of Diseases, 9th revision, Clinical Modification (ICD-9-CM).
Altogether, 23 006 case records were retrieved: bacterial or parasitic AGEs (001–007, and 008·0–008·5), viral AGEs (008·6–008·8), and AGEs of unknown etiology: ‘undetermined cause’ (009·0–009·3), ‘unspecified non-infectious AGE’ (558·9), or ‘diarrhea’ (787·91), any of which entered into any of the primary or secondary diagnosis fields (SDFs) [Reference Redondo, Cano and Simón4]. The Information and Sanitary Statistics Service of the Counsel for Health and Social Welfare in CLM provided us with the MBDS, specifically including the corresponding DRGs attributable to rotavirus. Weights and costs of DRGs were obtained from the cost accounting system Gescot®, run by the Economic Management Control Office of the General Secretary of SESCAM.
Definition of variables
Specified AGEs were defined by the presence of a specific ICD-9-CM code of bacterial, parasitic, or viral AGE in the primary diagnosis field of the MBDS, as well as by any of these codes in any SDFs accompanied by a principal diagnosis code of unknown etiology. In particular, rotaviruses (‘008·61’) and the other most commonly coded cause-specific AGEs were recovered this way (Salmonella ‘003·0–003·9,’ Campylobacter ‘008·43,’ adenovirus ‘008·62’, and other viral enteritis ‘008·8’). Non-specified (or non-typified) AGEs were those with a primary diagnosis code of unknown AGE and no specific code in their corresponding SDFs [Reference Redondo, Cano and Simón4].
LOS (in days) was estimated from the hospital admission and discharge dates. Extended LOS was defined ad hoc as any hospital stay over 3 days, as that was the median LOS for rotaviruses in CLM during the study period. Hospital readmission due to ‘the same process’ was defined as any hospitalization of the same patient twice in a 1-month period.
Medical costs were estimated as mean costs per DRG for each hospital and year of admission, in accordance with the Spanish norms that were operative throughout the study period (All Patient-DRG Groups, versions 18 and 23). These data are given in Supplementary Table S1. For the purposes of description and analysis, DRGs were clustered in three main groups: I. Non-bacterial AGE with complications (DRGs 813, 815); II. Non-bacterial AGE without complications (DRGs 777, 814, 816); and III. Highly weighted DRGs: admissions requiring surgical or other procedures as well as newborns requiring admission (DRGs 156, 468, 551, 613, 627, 628). Additional information on SESCAM's casuistic and functional indices can be found in Supplementary Table S2.
The 2007–2009 period was defined as the vaccine triennium, as it was the introductory and the longest time period in which both vaccines were commercially available in Spain simultaneously. For comparison purposes, the period between 2003 and 2005 was defined as the pre-vaccine period. Because only Rotarix® was made commercially available in Spain starting in July, 2006, that year was considered to be a transition period and was not included in our study.
Epidemiological factors, comorbidities and complications accompanying rotavirus-coded hospitalizations were retrieved from the SDFs and analyzed elsewhere [Reference Redondo-González and Tenías-Burillo29].
Statistical analysis
First, we categorized the total number of bed days corresponding to coded AGEs, both specific and non-specific, throughout the study period by age groups (<5 years or 5 years and up). For LOS, the mean and the s.d. were used. For extended LOS, absolute and relative frequencies were employed. For intra-group age (<5 or ⩾5, and total), comparisons of LOS and extended LOS between the main types of AGEs were calculated with the aid of the Student's t test and the χ 2 test, respectively. Pre- and post-vaccine LOS and extended LOS were described also separated into the two age groups, given the reported differences in prevalence of the different pathogens causing AGE that exist according to age [Reference Desai14]. Then, an adjusted analysis by age both of LOS and extended LOS was carried out for the most commonly coded community-acquired infectious AGEs requiring hospitalization (apart from rotaviruses) between the pre- and post-vaccine periods.
Afterwards, two estimate model approaches were specifically built for the stays of rotavirus-coded hospitalizations, simultaneously adjusting for those confounding or interacting (modifying) features that were identified in a previous stratified analysis of LOS and extended LOS between both 3-year periods: a multivariate linear regression analysis for LOS and a multivariate logistic regression analysis for extended LOS.
To quantify the magnitude of the association both for adjusted analysis by age of the most commonly coded AGEs and for multivariate regression analysis, we estimated the β-regression coefficient in the case of LOS and the odds ratio (OR) for extended LOS, both with their 95% confidence intervals (95% CI).
Additionally, a disaggregated analysis was made to explore the potential effect of vaccination on the decrease in LOS, both for hospitalized patients with rotavirus who presented comorbidities (perinatal problems, weight-height disorders, or co-infections), and for the most severe cases that needed intravenous rehydration.
Finally, analysis of the costs associated with DRGs attributable to rotavirus-coded hospitalizations in 2007–2009 were compared with those in 2003–2005. Prior to this, we corrected the annual average costs represented by each DRG in accordance with the Consumer Price Index for each year, obtained from an online application of the Spanish National Institute of Statistics. For each DRG group in each 3-year period, quality variables (readmission rates) and efficacy variables (mean hospital stay and DRG-based costs) were calculated. Pre–post comparisons of costs and stays by DRG group were analyzed with the independent Student's t test; the χ 2 test was used to compare readmission rates.
All analyses were performed using Stata 12 Statistical Software (StataCorp LP, TX); the level of significance (P) was assigned to values <0·05.
Ethics
The Ethical Review Board of the Service of Information and Sanitary Statistics of the Counseling of Health and Social Welfare in CLM approved this study.
RESULTS
A total of 17 415 AGEs were recovered throughout the study period; 5012 (29%) were coded as cause-specific. The most frequently coded agents were rotavirus (N = 1657; 33%), Salmonella spp. (N = 1581; 32%), undefined viral agents (N = 446; 9%), Campylobacter (N = 337; 7%), and adenovirus (N = 120; 2%).
Between 2003 and 2009, the average stay for ARGEs requiring hospitalization in children under 5 year was 3·79 ± 4·04 days; 45% of these ARGEs had an extended LOS (Table 1). Figure 1 shows the mean distribution of LOS further broken down into different ages. In the case of rotavirus, the longest average hospital stay was found in children under the age of 7 months (4·51 days, 95% CI 4·17–4·85). In the case of other specified AGEs as well as non-specified AGEs, the longest mean LOS was for children ⩾10 years (6·76 days, 95% CI 6·40–7·06 and 4·96 days, 95% CI 4·81–5·11, respectively). The mean LOS of patients with perinatal problems affected by ARGE was 3·34 days greater than for those without (95% CI 2·54–4·14, P < 0·0001). Similarly, LOS of patients with weight–height developmental disorders or with at least one concomitant infection in addition to ARGE was 1·12 and 1·00 days greater than for those without these additional problems (95% CI 0·49–1·74, P = 0·0005) and (95% CI 0·70–1·29, P < 0·0001), respectively.
Table 1. Comparison of LOS for all (specific and non-specific) coded AGEs in the 2003–2009 period by age groups (Source: MBDS, Castile-La Mancha, Spain*)
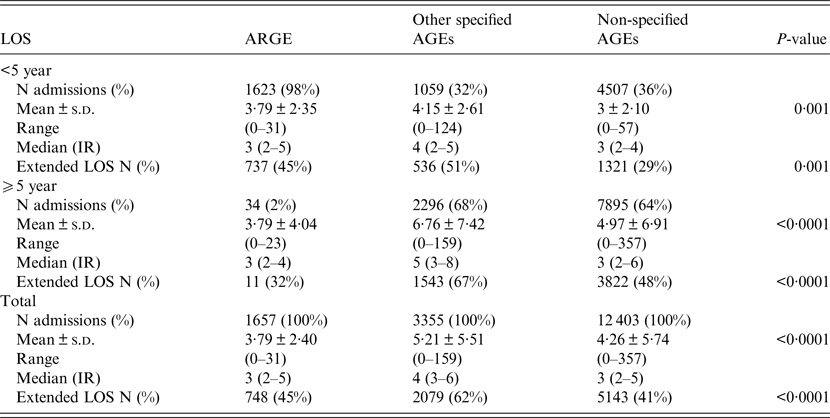
IR, interquartile range.
* The assumption of normality by age group for the three types of AGEs was confirmed with the aid of the Shapiro–Wilks test.
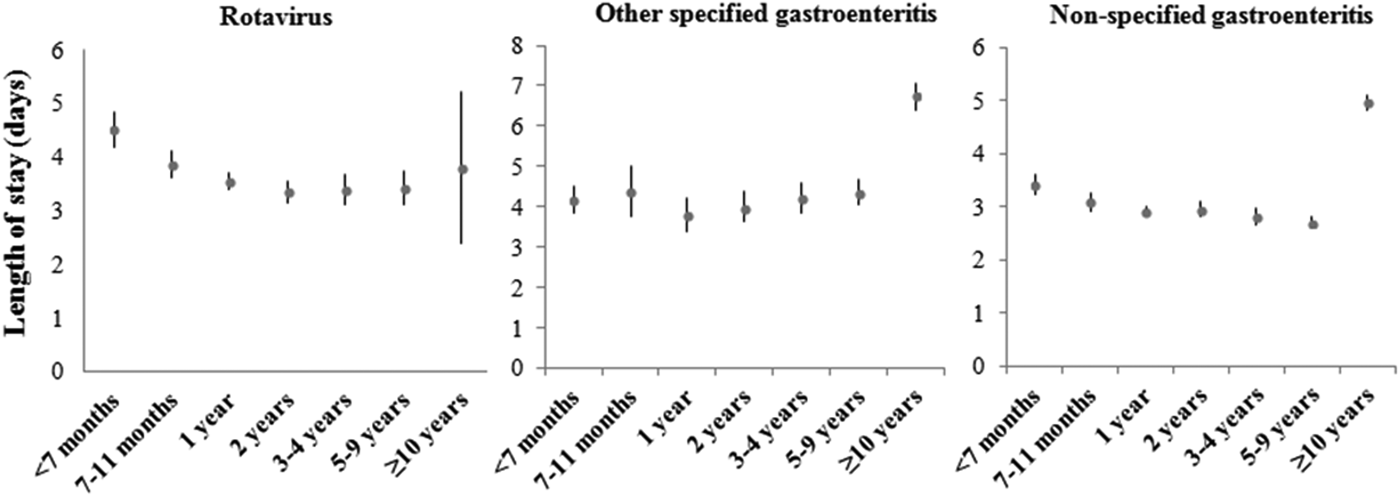
Fig. 1. Distribution of means for LOS (in days) with their 95% CI, by age groups, for rotavirus and other specific and non-specific coded AGEs. Period 2003–2009. Source: MBDS of CLM, Spain.
Figure 2 represents annual distribution of LOS and extended LOS for the main groups of gastroenteritis overall, according to their codification: a general downward trend of LOS during the vaccine period can be observed in all cases, even in non-specified AGEs. However, as shown in Table 2, a significant decline in the mean LOS was only found for rotavirus (β-coef. = −0·43, 95% CI −0·68 to −0·17) and Campylobacter (−1·35, 95% CI −2·39 to −0·30). Unlike rotaviruses, LOS of hospitalizations due to Campylobacter was 4·22 days longer in patients ⩾5 years (95% CI 3·15–5·28, P < 0·0001). With respect to other non-specified AGEs, LOS experienced a non-significant decrease in children <5 years (−0·07, 95% CI −0·43 to 0·28, P = 0·693) during the vaccine period, but increased in children ⩾5 years of age (0·44; 95% CI 0·17–0·70; P = 0·001). Extended LOS also decreased significantly in the case of rotavirus (OR 0·62, 95% CI 0·50–0·76) and Campylobacter (OR 0·32, 95% CI 0·19–0·55) (Table 3). Again, the mean extended LOS for Campylobacter was four times higher in patients ⩾5 year (95% CI 2·42–7·49, P < 0·0001). During the vaccine period, extended LOS for ‘other non-specified AGEs’ decreased significantly in children <5 year (OR 0·840; 95% CI 0·736–0·958, P = 0·010), but increased non-significantly in those ⩾5 (OR 1·05; 95% CI 0·96–1·15, P = 0·254).

Fig. 2. Distribution of both the LOS in days (graph above) and the percentage of extended LOS (graph below), for all (specific and non-specific) coded AGEs by year. Source: MBDS of CLM, Spain.
Table 2. Adjusted analysis of LOS (in days) by age groups for the most common codified community-acquired infectious AGEs requiring hospitalization between the pre (2003–2005)- and post-vaccine (2007–2009) periods in CLM, Spain*
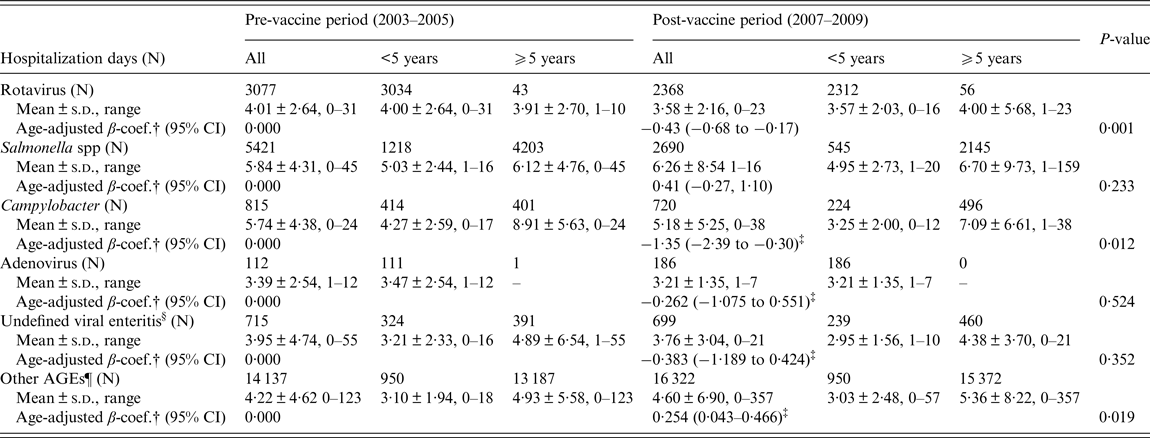
* No significant interaction of ‘age group’ (⩾5/<5 years) was found in the association between LOS and 3-year period in known AGEs (p-rotavirus = 0·595; p-salmonella = 0·404; p-campylobacter = 0·460; p-adenovirus = non-evaluatable; p-undefined viral enteritis = 0·762); but an interaction was found in the case of unknown AGEs (p-other AGEs=0·025).
† β regression coefficient adjusted by age group (⩾5/<5 years), comparing LOS (in days) between post- and pre-vaccine periods.
‡ Age was a confounding factor for the association between LOS and the two 3-year periods: LOS of Campylobacter (β-coef.⩾5/<5 = 4·22; 95% CI 3·15–5·28; P<0·0001); LOS of adenovirus (β-coef.⩾5/<5 = non-evaluatable); LOS of other viral enteritis (β-coef.⩾5/<5 = 1·91; 95% CI 0·35–10·54; P = 0·457).
§ Undefined viral enteritis: ICD-9-CM code ‘008·8’
¶ Ninety-eight percent of other AGEs were coded as non-specified (or non-typified) AGEs in the MBDS.
Table 3. Adjusted analysis (by age group) of extended LOS for the most commonly coded community-acquired infectious AGE requiring hospitalization between the pre (2003–2005)- and post-vaccine (2007–2009) periods in CLM, Spain*
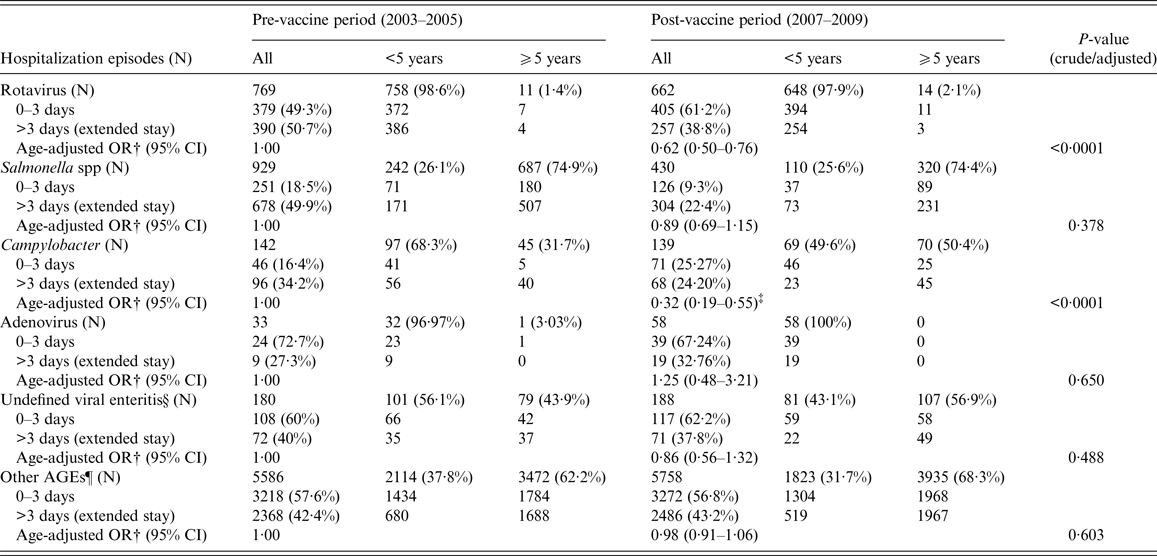
N, number of hospital admissions (hospital episodes).
* No significant interaction of ‘age group’ (⩾5/<5 years) was found in the association between extended LOS and 3-year period in known AGEs (p-rotavirus = 0·772; p-salmonella = 0·684; p-campylobacter = 0·438; p-adenovirus = non-evaluatable; p-undefined viral enteritis = 0·403); but an interaction was found in the case of unknown AGEs (p-other AGEs = 0·005).
† OR adjusted by age group (⩾5/<5 years), comparing extended LOS (>3 days) between post- and pre-vaccine periods.
‡ Age was a confounding factor for the association between extended LOS of Campylobacter and the two 3-year periods: OR⩾5/<5 = 4·40; 95% CI 2·42–7·49; P < 0·0001.
§ Undefined viral enteritis: ICD-9-CM code ‘008·8’.
¶Ninety-eight percent of other AGEs were coded as non-specified (or non-typified) AGEs in the MBDS.
After a stratified analysis of decrease in LOS for rotavirus hospitalizations, no interacting variables were found, and only ‘provinces of residence’ turned out to be a confounding variable in such decrease: crude β-coef. = −0·42 (95% CI −0·68 to −0·17, P = 0·001); and adjusted β-coef. by provinces of residence = −0·39, (95% CI −0·64 to −0·14, P = 0·002). The consequent multivariate linear regression analysis including ‘provinces of residence’ showed that the decrease in LOS was only significant in Ciudad Real province (β-coef. = −0·31, 95% CI −0·609 to −0·140, P = 0·040) and Guadalajara province (β-coef. = −0·99, 95% CI −1·56 to −0·43, P = 0·001). In the stratified analysis for extended LOS, we did not find any variable that confounded or modified the decrease of extended LOS in the vaccination period.
We found non-significant decrease in the LOS for rotavirus hospitalizations with concomitant perinatal problems (P = 0·135) or weight–height disorders (P = 0·822) in 2007–2009; but there was a significant decrease in LOS of ARGEs with at least one concomitant infection (β-coef. = −0·66, 95% CI −1·25 to −0·075, P = 0·027) and in those requiring intravenous rehydration (β-coef. = −1·37, 95% CI −2·56 to −0·17, P = 0·025).
Global costs attributable to rotavirus hospitalizations went down approximately €243·65 per patient (95% CI −365·18 to −123·18, P = 0·0001) during the vaccine period, with a 1·5% decrease (95% CI −2·8 to −0·2%) in the readmission rate (P = 0·0318) (Table 4). A real decrease of €2268·83 per patient was observed for more highly weighted DRGs (95% CI −4097·87 to −379·76; P = 0·0204), since the mean cost for DRGs corresponding to groups I and II rose by €476·79 per patient in 2007–2009 (95% CI 408·98 to 544·61; P ⩽0·001). Besides, the average decrease in LOS for DRGs owing to group III was 3·56 days (95% CI −5·28 to −1·84; P < 0·0001).
Table 4. Evaluation of change in mean LOS and costs attributable to hospital admissions caused by rotavirus in CLM, Spain (Sources: MBDS, 2003–2009; Cost Accounting System Gescot®, Economic Management Control, General Secretary of the Health Service of Castile-La Mancha*)

* Groups have been defined according to individual DRG costs (Supplementary Table S3): I (1500–2200€); II (<1500€); III (>2200€).
† Each of these GRDs represented a median cost for the entire study period that was over €2000 per hospital admission.
DISCUSSION
After the 2007–2009 period with a mean vaccine coverage of 34% in CLM (from 18% in 2007 to 44% in 2009), we found a 14% decrease in the median rate of rotavirus hospitalizations (95% CI 4–22) [Reference Redondo, Cano and Simón4] leading to a modest overall savings of €26 071 for SESCAM, which were largely attributable to a fall in cases with higher DRG weights (representing nearly €2300 per patient). While the overall mean LOS of rotavirus hospitalizations fell by almost half a day (a 10% decrease); the mean LOS for the highest DRGs fell by almost 4 days (an eightfold higher decrease). Further, the decrease in LOS was 7% and 24% greater for ARGEs accompanied by at least one concomitant infection or requiring intravenous rehydration, respectively; and extended LOS fell by 38%.
A prospective observational study conducted from October 2008 to June 2009 covering the regions of North-west Spain concluded that indirect societal costs due to ARGE in children up to 5 years old (~192·7€ per family) were 1·74-fold higher compared with AGEs of other etiologies [Reference Bouzón-Alejandro30]. Although the aim of our work was not determining societal costs, the projection of such value to the 709 hospital admissions avoided during the vaccination period would allow us to estimate an approximate additional saving of €136 624; of which, €85 364 would be due to absenteeism.
Extended LOS for non-specified AGEs decreased significantly by 16% in children <5 year, many cases of which could actually be attributed to rotavirus, as some similarities were observed in their incidence when they were categorized by age groups [Reference Redondo, Cano and Simón4]. This hypothesis concerning an under-codification of rotaviruses was bolstered by the similarities observed in the seasonality of ARGEs and non-typified AGEs [Reference Redondo, Cano and Simón4, Reference Gil31]. In fact, it is a well-known fact that approximately 28% of ARGEs are underdiagnosed in the MBDS [Reference Mast8]. In this respect, the LOS trend-line for non-specified AGEs in Figure 2 seems very similar to that of rotaviruses, particularly from 2007 onwards, with the lines representing the percentages of extended LOS for rotaviruses and those for non-specified AGEs overlapping in this period. Additionally, the median LOS for both ARGEs and non-specified AGEs between 2003 and 2009 was the same (Table 1).
Diarrhea of undetermined etiology had already been taken into account for analyzing in parallel with rotavirus hospitalizations in Spain [Reference Gil-Prieto19], but neither the impact in LOS nor the costs that could be attributed to rotaviruses were calculated. We had previously estimated that 54% of the registries coded with a principal diagnosis of AGE are non-typified in children under 5 years of age, so it was worth analyzing their behavior in relation to rotaviruses. Others have also emphasized that underestimation of ARGE hospitalizations may be also due to the fact that estimations are usually made using only the principal diagnosis [Reference Marocco32]; but we have only estimated 17 cases with a primary diagnosis code of non-typified AGE and a specific code of rotavirus in their corresponding SDFs [Reference Redondo, Cano and Simón4]. Anyway, poor classification of non-specified gastroenteritis events (including rotavirus cases in this group) can only lead to a dilution of the potential impact of the rotavirus vaccine both in terms of costs and stays (bias toward null value or non-differential classification bias).
In this study we analyzed the decrease of stays apart from that of costs because in SESCAM the price of each DRG is not calculated by the cost attributable to each patient individually, but by multiplying each DRG weight by the ‘weight unit’ cost (or Hospital Complexity Unit (HCU) cost) owing to the hospitalization ‘Homogeneous Functional Group’ (HFG) of a department: HCU cost of a department = total cost of the hospitalization HFG of the department/total weight of the hospitalization HFG of the department. The calculation of the total weight of the hospitalization HFG is made independently of the stays as Σ (unit weight of each DRG × number of hospital discharges with that DRG); as the hospitalization department would maintain, with rare exceptions, a full stay throughout the year for whatever reason of admission. Therefore, according to this management system, the average stay would not influence directly the HCU cost of a department. Although LOS does not directly influence the costs, the LOS decrease would have an impact on social costs by reducing parental absenteeism, while improving the efficiency of the neonatal-pediatric department, allowing other patients to be hospitalized and thus decreasing the waiting lists.
The decrease in LOS for ARGEs was only significant in Ciudad Real and Guadalajara provinces. The vaccination coverage of both were the largest and the ones that increased the most during the triennium of vaccination, together with Albacete, although here the decrease in LOS did not become significant (Supplementary Table S4). One reason why it did not happen in Albacete could be a misclassification in exposure variable (data provided by laboratories come from distributed, but not administered vaccines, so we do not know to what extent immunization schedules were administered completely); and on the other hand, that hospital stays depend on the own department and/or intra-hospital bed management and its consequent efficiency.
DRGs are universally recognized tools used to optimize Health Systems [Reference Rivero22]. However, to date, we only found two DRG-based studies in Spain, investigating the cost-effectiveness for infectious diseases, both not dealing with vaccines [Reference Gómez23, Reference Arteaga-Rodríguez33]. According to a national study, the introduction of universal vaccination with RotaTeq® would not be cost-effective in Spain [Reference Imaz Iglesia34]. Notwithstanding, more research was needed on real cost savings. We only observed a significant cost decrease for more highly weighted DRGs accompanied by a significant decline in LOS of almost four days, despite the number of cases did not decrease (Table 4). On the contrary, the major descent in the number of hospitalizations occurred in group II, with a significant decrease in LOS by half a day, but with an increased cost. Regarding group I, the costs increased, but the mean stay did not change. Therefore, in the vaccination period, the less complicated hospitalizations due to rotavirus (group II) fell by 20% (from 604 to 481), at the cost of only an 11% increase (from 145 to 160) in hospitalizations of ARGEs with complications (group I). If unit mean costs owing to each I, II and III DRG groups had not increased so much in 2007–2009, overall savings we have estimated would have been even greater. It seems that a probable lower efficiency in the context of a greater complexity of the own hospitalization units for rotavirus (pediatrics and neonatology) over the years might have directly expanded the mean costs estimations by DRG in 2007–2009. That is because the increase in costs would have exceeded the increase in complexity. The hypothesis of a higher complexity in 2007–2009 is because the case mix (or casuistic) index (CIX) from pediatric and neonatology units of SESCAM indicates that the casuistry of the analyzed series in the vaccine period (CIX 0·58) was somewhat higher than that of the pre-vaccine period (CIX 0·37) (Supplementary Table S2). The supposition of a probable lower efficiency is because the performance or (functional) index of both 3-year periods reveals that those units of SESCAM attended its patients with an average mean stay similar to that of Spain; despite of its complexity was significantly lower than that of the SNHS (Supplementary Table S2).
The selling price of Rotateq® for complete immunization to the public in pharmacies at the end of the study period was about €207, while the selling price of the reference laboratory was €133·5. In Spain, there is no option that the cost of vaccines not included in the universal national immunization program will be partially reimbursed by the Government. That is, the rotavirus vaccination is fully self-financed by consumers, which makes it difficult to acquire for families facing economic difficulties. The selling price of Rotateq® for complete immunization to the public in pharmacies at the end of the study period was about €207, while the selling price of the reference laboratory was €133·5. Then, while savings of €26 071 due to the decrease (by 107 cases) in rotavirus hospitalizations during the 2007–2009 period was a consequence of patients self-financing the vaccination at a cost of €22 149(107*207) for them, the vaccination cost for the SNHS would actually be lower, namely €14 285(107*134). If the actual level of 34% coverage reached in 2007–2009 had been provided by a public immunization program at that lower per unit cost, the vaccine would have been cost-effective because the expense of the vaccination for the SNHS represents €11 786·05 less than the overall savings we have estimated. Furthermore, a national immunization program would let reach a higher coverage and so, get more sustained saving over the time.
Long-term trends in Europe suggest an increase of campylobacteriosis [Reference Schielke, Rosner and Stark35, Reference Maraki36]. We discovered a decrease by 28% in children under 5 years of age, and LOS and extended LOS for Campylobacter decreased significantly by almost 1½ days and by 68%, respectively. One reason for this decrease could be simultaneous co-infection with rotavirus. Although viruses are the most commonly reported agents of gastrointestinal co-infections accompanying rotavirus [Reference Wolak, Wałaszek and Dobroś37, Reference Valentini38], Campylobacter spp. is the most frequent cause of enteropathogenic concomitant AGE for ARGEs, present in up to 19% of cases [Reference Valentini38]. While co-occurrence of rotavirus and Campylobacter was coded in 5% of hospitalizations in children under 5 years of age during 2003–2005, this co-infection was not present during 2007–2009. This would contribute to the decrease observed in stays for campylobacteriosis during 2007–2009, as co-infections involve an increased assistance during hospitalization [Reference Li39]. Although very little is known about the role of interaction between pathogens in causing diarrhea, a recent study has reported that the co-occurrence of rotavirus and Campylobacter jejuni only occurs in symptomatic children (P < 0·0001) and that Salmonella usually does co-occur with norovirus better than with rotavirus [Reference Valentini38].
Our study has some limitations. Although MBDS was designed for financial management purposes, certain incremental change in health costs over time is presumed to be inevitable. Despite we took into consideration the yearly Consumer Price Index, other factors influencing the incremental mean unit costs of DRGs over the years may have contributed to an underestimation of the real savings. The recognition of the health workers’ Professional Career status that began in 2006 is a good example, which of course might have entailed an increase in the budget set to health personnel salaries. We also commented above a lower efficiency of pediatric and neonatology units, which means incremental costs exceeding an incremental complexity. Actually, there was an increase in public health expenditure of SESCAM despite the economic crisis that started in Spain in 2008 (Supplementary Fig. S1). There is also the possibility of underestimation in the recruitment of cases from the discharge database. The dependency of our estimations on accuracy coding remains a problem when comparing and interpreting trends in the five provinces of CLM, even though the variable ‘provinces’ is a field always fulfilled in the MBDS. In this sense, we found some differences in the decrease in LOS and in the incidence rate by provinces [Reference Redondo, Cano and Simón4], despite we found no province-specific differences in vaccine coverage (P = 0·599). Finally, it is well known that the true burden of community-acquired rotaviruses is generally misclassified when estimated in hospital databases through ICD-9-CM [Reference Mast8], with a maximum sensitivity of 47% [Reference Hsu40]; albeit this underestimation that is supposed to affect equally both periods of comparison would not influence the estimation of changes occurring between them.
The biggest strength is that this research constitutes the first national study published using the DRG system to evaluate cost savings after a period of vaccine implementation. The 2007–2009 study period coincided with the above mentioned economic crisis, therefore some LOS descent findings may be expected to be due to economic constrains, more than the vaccines. But the decrease in rotavirus hospitalization costs gives meaning to the trend to a lower LOS that only became significant for rotavirus and Campylobacter (the last, the most frequent cause of enteropathogenic concomitant AGE for ARGEs), which would reflect a modest vaccine impact more than health policy changes or an improvement in the AGE management.
In summary, the actual modest decrease in hospital costs after the 3-year period of simultaneous Rotarix® and Rotateq® availability in Spain was due to cases with higher DRG weights; and the decrease in LOS would represent a faster recovery of such complex cases or even of more moderate-to-mild cases hospitalized post-introduction. Alongside this, the consequent savings in social costs according to previous studies might have been even fivefold higher than hospital savings; to which we would even have to add a specific saving in social costs derived from a lower parents’ absenteeism, as a consequence of the average stay decrease in the vaccination period. Despite a universal rotavirus vaccination program has been discarded in Spain for not being cost-effective overall, the present study provides a first step toward re-evaluating its implementation, regarding its potential high impact on both at-risk children and societal costs.
SUPPLEMENTARY MATERIAL
The supplementary material for this article can be found at https://doi.org/10.1017/S0950268817000620
ACKNOWLEDGEMENTS
The authors would like to thank Rosario Valverde Vaquero and Inés Ruano del Cerro, from the Economic Management Control, General Secretary of the Health Service of Castile-La Mancha in Toledo, who provided disaggregated data on costs by years and hospitals, allowing us to perform exact estimations of costs. The authors have no financial relationships to disclose.
DECLARATION OF INTEREST
None.









