INTRODUCTION
Latvia has consistently had one of the highest rates of multidrug-resistant tuberculosis (MDR-TB) in the world, affecting 11% of all new TB patients and 36% of patients previously treated for TB in 2005 [1]. Latvia is currently listed as 26th of 27 high-burden MDR-TB countries in the world [1]. In response to this crisis recognized over a decade ago, the National TB control programme in Latvia has adopted several internationally recognized TB control strategies. In 1996, the directly observed treatment, short course (DOTS) strategy was implemented nationwide, but was soon recognized as being insufficient to impact increasing rates of drug-resistant TB. Therefore in 1998, the national programme adopted an enhanced strategy for MDR-TB, at the time referred to as DOTS-plus, which included: enhanced MDR-TB case detection, individualized treatment regimens using second-line drugs, an individualized approach to case management, standardized recording and reporting, routine treatment outcome analysis, and a consistent and reliable drug supply. Since the implementation of this strategy treatment outcomes have improved markedly; however, MDR-TB rates remain high and are still a concern. There is a clear need to identify modifiable factors that impact MDR-TB patient outcomes.
The interrelationship between nutritional status and infectious diseases is well recognized [Reference Scrimshaw2], and the synergy between poor nutrition and TB dates back to early descriptions of the disease as phthisis, Greek for ‘consumption’, literally meaning wasting of the body [Reference Davis, Reichman and Hershfield3]. Sanatoria, the primary mode of treatment prior to the advent of effective chemotherapy in the mid-20th century, emphasized the need for proper nutrition as a component of TB treatment.
Several previous studies have suggested that nutritional status may increase susceptibility to TB disease [Reference Macallan4, Reference van Lettow, Fawzi and Semba5], and in those treated, being underweight at the time of diagnosis has been demonstrated to increase relapse rates [Reference Khan6] and accelerate time to death [Reference Zacharia7]. However, to our knowledge there are no previous studies that have evaluated the impact of nutritional status in patients with MDR-TB. The current study aimed to elucidate the association between nutritional status and clinical presentation, clinical course, and mortality in patients treated for MDR-TB in Latvia.
METHODS
A retrospective study was conducted to include all adult (⩾18 years) patients who were diagnosed with pulmonary MDR-TB and registered in the National MDR-TB database in Latvia, and who initiated individualized MDR-TB treatment between January 2000 and June 2004. The second-line drugs available for use during this time included amoxicillin, capreomycin, clarithromycin, cycloserine, kanamycin, ofloxacin, p-aminosalicylic acid, protionamide, and thioacetazone. The electronic MDR-TB database was designed specifically by CDC for use in surveillance of MDR-TB in Latvia as well as being a clinical treatment record. It records hospital and ambulatory patient records, bacteriological laboratory reports, and outcome data during the patient's entire course of treatment. All patients underwent an initial clinical examination, which included obtaining socio-demographic information, medical and TB treatment history, a biological sputum specimen for smear, culture, and drug-susceptibility testing, and a chest X-ray. Patients were evaluated monthly to assess treatment side-effects and to collect additional biological specimens to test for smear and culture and assign interim and final treatment outcomes.
Nutritional status
Body mass index (BMI) was calculated for each patient based on weight and height at the time of MDR-TB diagnosis [BMI=weight (kg)/height (m2)]. Nutritional status was defined by the international standards of the World Health Organization, which designates the cut-off for underweight as BMI <18·5; a BMI of ⩾18·5 is considered normal or overweight [8].
Outcomes
Prevalence of clinical symptoms, smear and culture status, resistance patterns, and severity of TB disease on chest X-ray were assessed at the time of initial clinical presentation for MDR-TB. Over the course of treatment, side-effects and treatment modifications were recorded. Final treatment outcomes were assigned according to international guidelines: as cured, completed treatment, defaulted from treatment; treatment failure, and death [9]. Date of death was obtained for patients who died during the course of treatment.
Statistical analyses
Logistic regression analyses were used to assess the cross-sectional association between baseline nutritional status and initial clinical presentation. Generalized linear models were used to evaluate the association between baseline nutritional status and number of side-effects and treatment modifications over the course of treatment, with relative risk as the measure of association. Cox proportional hazards models were used to estimate the association between baseline nutritional status and risk for incident events that occurred over the course of treatment and for final treatment outcomes and mortality. Overall treatment failure was considered as defaulting from treatment, failing treatment, or dying during the course of treatment. For the final treatment outcomes, time was calculated as the number of days from initiation of MDR-TB treatment to either treatment failure or death; all other outcomes were censored. Patients who defaulted from treatment were considered censored at the time of their last clinic visit.
For all of the models, the association between nutritional status at baseline and the outcome of interest was initially explored using univariate analyses. All other socio-demographic and clinical characteristics of patients were also examined for an association with the outcome of interest using univariate analyses. All multivariate models were constructed using forward selection, using nutritional status as the key exposure, and considering each additional variable that met one of the following criteria: (1) was previously cited in the medical literature as having an important association with the outcome; (2) had a biologically plausible association with the outcome; or (3) was initially identified as having a significant univariate association with the outcome of interest at α<0·20. Age was included in all models irrespective of significance; other variables were only retained in the final multivariate model if they had α<0·05. Collinearity between variables and effect modification was also assessed. When collinearity was present between variables, the variable that was the most robust predictor of the outcome under consideration was retained in the final multivariate model. All analyses were conducted using Stata version 10.0 (StataCorp, USA).
Ethical review
This analysis was determined to be a programmatic evaluation and was approved as non-human subjects research by the U.S. Centers for Disease Control and Prevention. The protocol was approved by the Latvian Ministry of Health.
RESULTS
Between January 2000 and December 2004, a total of 1019 adult pulmonary MDR-TB patients initiated individualized MDR-TB treatment. Of these patients, 12 and 11 patients had missing baseline measurements of weight and height, respectively. One additional patient transferred out of the country and was missing final treatment outcome status. A total of 995 adult patients were therefore included in the current analysis.
Of the 995 patients, 401 (40%) were classified as having primary drug resistance and 594 (60%) were considered to have acquired drug resistance. Thirty-seven percent had no previous history of TB treatment, but 51% had been previously treated for drug-susceptible TB, and 12% had a history of MDR-TB treatment. In total, 199 (20%) patients were considered underweight (BMI <18·5) at the time of MDR-TB diagnosis.
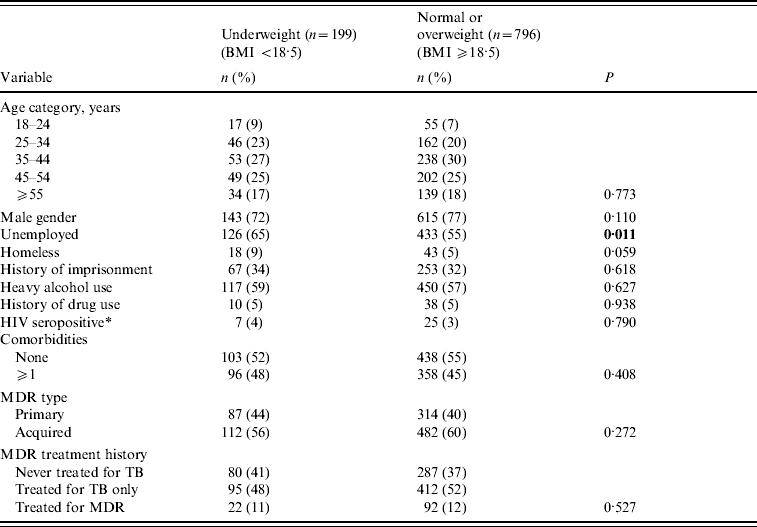
Patients who were underweight were similar in age and gender distribution as patients who were normal or overweight, but were more likely to be unemployed (Table 1). There were no differences between underweight and normal weight patients regarding history of heavy alcohol use, drug use, or current HIV infection. Underweight patients were not more likely to have a medical history of diabetes, cardiovascular disease, liver disease, other lung disease, or bowel disease; nutritional status was not significantly associated with the overall presence of one or more comorbid conditions. In addition, patients who were underweight had similar medical and TB treatment histories as patients that were of normal or overweight.
Table 1. Baseline socio-demographic and treatment history of pulmonary multidrug-resistant (MDR)-TB patients by level of nutritional status (n=995)

Bold values indicate statistical significance at P<0.05.
Statistical comparisons across categories by χ2 or Fisher's exact test (if cell n<5).
* HIV testing performed on 814/995 (82%) patients.
Clinical presentation
Patients who were underweight were significantly more likely to be smear positive, culture positive, have cavitation evident on chest X-ray, and to report fever, cough, and weight loss than patients who were normal or overweight (Table 2). In addition they were more likely to present with more advanced disease, as evidenced by a higher culture colony count [⩾3 colonies: odds ratio (OR) 2·7, 95% confidence interval (CI) 1·6–4·5, P<0·001] and bilateral cavitation (OR 2·8, 95% CI 1·8–4·3, P<0·001). There was no association between nutritional status and level of resistance to first- or second-line anti-TB drugs. Overall, the median number of days between the time of MDR-TB diagnosis to the time of MDR-TB treatment initiation was significantly less in patients who were underweight compared to patients who were normal or overweight [7 days (interquartile range (IQR) 1–23 days) vs. 13 days (IQR 3–29 days); P<0·001]. However, the difference in medians was no longer significant after accounting for initial sputum status (P=0·125); drug-susceptibility testing was performed with more rapid tests using liquid media for patients with more severe clinical presentation.
Table 2. Baseline disease characteristics by level of nutritional status (n=995)
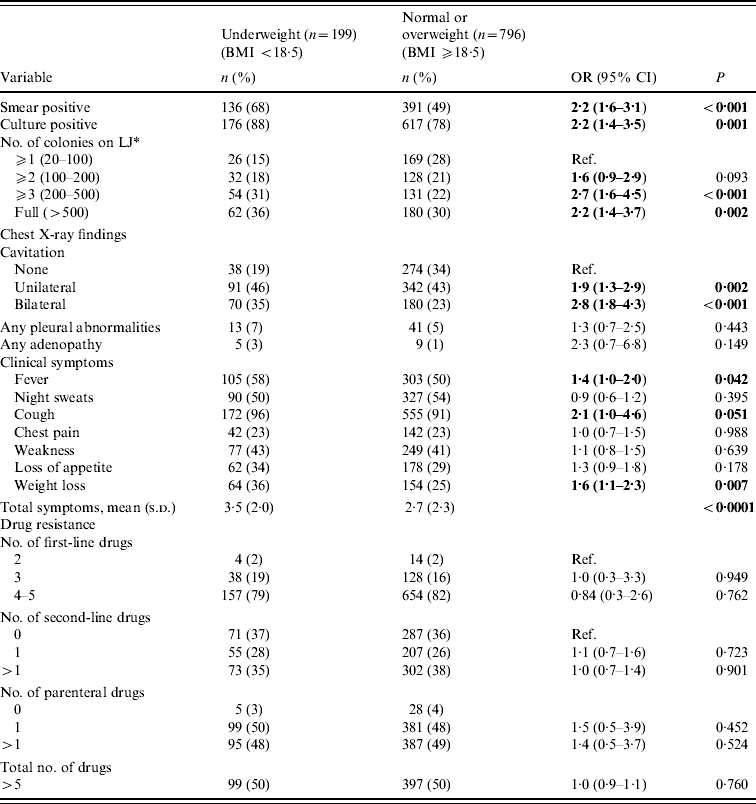
BMI, Body mass index; OR, odds ratio; CI, confidence interval; LJ, Lowenstein–Jensen solid media.
Bold values indicate statistical significance at P<0.05.
* Colony count only in patients who were culture positive (n=793).
In multivariate models adjusting for age, being underweight at baseline was independently associated with culture positivity [adjusted odds ratio (aOR) 1·7, 95% CI 1·1–2·9, P=0·037], extent of cavitation (unilateral: aOR 1·6, 95% CI 1·0–2·6, P=0·04; bilateral: aOR 2·1, 95% CI 1·3–3·5, P=0·002), and reporting recent weight loss (aOR 1·5, 95% CI 1·0–2·1, P=0·04) (Table 3). Findings were similar when the analyses were restricted to patients that had not been previously treated for TB, and to patients that had been previously treated for drug-susceptible TB but not MDR-TB (data not shown).
Table 3. Association between baseline sociodemographic and disease characteristics and baseline nutritional status* (n=995)

aOR, Adjusted odds ratio; CI, confidence interval.
Bold values indicate statistical significance at P<0.05.
* All measures of association compare patients that were underweight to patients that were normal or overweight at the time of MDR-TB diagnosis.
Clinical course
Over two-thirds of patients (715/995, 72%) experienced at least one side-effect, with each patient experiencing a median of three side-effects (IQR 0–6) over the course of treatment. The most common side-effects reported by patients were nausea (54%), vomiting (37%), dizziness (23%), abdominal pain (22%), and diarrhea (19%). Patients who experienced ⩾3 side-effects over the course of treatment were more likely to be underweight at the time of initiating treatment (aOR 1·5, 95% CI 1·1–2·1, P=0·018), after adjusting for age, culture status, and extent of cavitation at baseline. Being underweight was not significantly associated with risk for permanent drug discontinuation, treatment interruptions, or total number of days a patient missed a treatment dose.
For the 793 patients that were culture positive at the initial diagnosis, 557 (70%) culture-converted over the course of follow-up (66% and 71% in underweight and normal weight patients, respectively). Being underweight at baseline was not significantly associated with culture conversion [hazard ratio (HR) 1·1, 95% CI 0·9–1·3, P=0·49].
Final treatment outcome and mortality
A total of 675 patients (69%) were cured or completed treatment, 141 (14%) defaulted from treatment, 124 (13%) were considered treatment failures, and 55 died (5·5%) over the treatment course. No significant association was identified between underweight and overall treatment failure, which compares patients who default, fail treatment, or die during the treatment course to patients that were cured or completed treatment (HR 1·2, 95% CI 0·9–1·5, P=0·23).
Overall, patients who were underweight at the time of MDR-TB diagnosis had almost twice the risk of death compared to patients who were normal or overweight [adjusted hazard ratio (aHR) 1·9, 95% CI 1·1–3·5, P=0·03], after adjusting for age, bilateral cavitation, and category of previous TB treatment. Upon further inspection in analyses stratified by previous TB treatment category, it appeared that the association between nutritional status and death was largely driven by patients who were treatment-naive at the time of the current MDR-TB diagnosis (aHR 3·2, 95% CI 1·1–9·0, P=0·04). The association was not significant within strata of patients previously treated for drug-susceptible TB (aHR 1·6, 95% CI 0·7–3·7, P=0·28) nor patients with previous MDR-TB treatment exposure (aHR 2·1, 95% CI 0·5–8·1, P=0·29).
CONCLUSIONS
Twenty percent of patients who initiated MDR-TB treatment between 2000 and 2004 in Latvia were underweight at the time of MDR-TB diagnosis. Although nutritional status at the time of MDR-TB diagnosis does not allow for a conclusion to be made about the temporal association between nutrition and disease, several other studies have reported poorer nutritional status in TB patients compared to healthy controls [Reference van Lettow, Fawzi and Semba5]. Overall, being underweight was significantly associated with a more severe clinical presentation, as evidenced by a higher number of symptoms, and a higher proportion of patients that were smear positive, and who had notable cavitation on chest X-ray. Patients who were underweight were more likely to report recent weight loss, a finding consistent with a previous survey conducted in South Africa that identified weight loss as a significant indicator of more advanced TB disease (OR 14·2, 95% CI 4·4–46·2) [Reference den Boon10]. In addition, underweight patients in the current study experienced a higher number of side-effects over the course of treatment. A study conducted in the 1960s in India comparing clinical outcomes in TB patients treated in either a sanatorium with a balanced diet or at home with a poorer diet found that patients receiving better nutrition had an accelerated time to smear conversion and resolution of radiographic abnormalities, concomitant with weight gain, compared to patients consuming a poorer diet [Reference Ramakrishnan11]. Data from a recent clinical trial in patients with active TB showed being underweight at the time of diagnosis, as measured by BMI, to be related to an increased risk for relapse [Reference Khan6].
In our study, patients who were underweight also had an almost twofold higher risk for death compared to patients who were of normal or overweight at the time of MDR-TB diagnosis. A previous study conducted in drug-susceptible TB patients in India identified lower weight as a significant risk factor for mortality (aOR 3·8, 95% CI 1·9–7·8) [Reference Santha12], and a study conducted in TB patients co-infected with HIV in Malawi found that patients with moderate or severe malnutrition were more likely to die within the first 4 weeks of TB treatment (OR 1·8, 95% CI 1·1–2·7) [Reference Zacharia7]. Of note, we found that the risk association between being underweight and death was greatest in patients who had not previously been treated for TB. These patients may have harboured TB illness for a longer duration before presenting for medical care, and therefore the disease may have been more advanced at the time of diagnosis. In our cohort, patients who had not been previously treated did not a have a higher proportion of smear-positive cases or extensive cavitation on chest X-ray compared to patients who had been previously treated for TB or MDR-TB. However, patients who had not been previously treated had significantly more clinical symptoms at the time of diagnosis [mean 3·3 symptoms vs. 3·1 (previous TB treatment) and 1·3 (previous MDR-TB treatment) symptoms, P<0·001].
This study critically examined the role of nutritional status at diagnosis and treatment course in patients with MDR-TB. However, as with any study ours is not without limitations. We restricted our analysis to adult pulmonary MDR-TB patients. Although there were only 19 cases of extrapulmonary MDR-TB and eight cases of MDR-TB in children recorded during this time period, our sample comprised the majority of registered MDR-TB patients in Latvia. This analysis was conducted on programmatic data compiled as part of a national TB registry, and may not be representative of all persons in the community that have MDR-TB. There is some research that suggests patients identified through surveillance may be in the later stages of the disease course due to clinical symptoms prompting a visit to a health provider. However, case detection rates for TB in Latvia are quite high, estimated at 89% [1], and drug-susceptibility testing is routinely performed on all TB patients before the initiation of treatment. Data collected for programmatic surveillance purposes or based on medical chart reviews are often incomplete for key variables of interest. HIV testing was not routine for all patients, and information on specific comorbidities was not systematically assessed; therefore there is potential for misclassification of these variables due to missing or incomplete data. However, in this cohort, there were few patients that were missing the key exposure of interest (BMI based on weight and height) and only one patient missing the final treatment outcome. Next, we used BMI as our measure of malnutrition. It is a crude measure that may not be sensitive enough to differentiate groups that may have different risk profiles for adverse events, nor specific enough to reflect components of nutritional status that may impact risk. However, BMI has been demonstrated to be a risk factor for early mortality in TB patients co-infected with HIV [Reference Zacharia7]. BMI has also been shown to be related to micronutrient deficiencies in persons with TB [Reference Karyadi13]. In addition, weight and height were only measured at the time of diagnosis. We did not have any assessment of whether persons who were underweight were underweight prior to TB infection and disease, and therefore cannot establish temporality. It is important to recognize that the association of low BMI and more severe clinical presentation at the time of diagnosis does not implicate a causal relationship. However, patients who were underweight reported weight loss significantly more often than persons of normal or overweight during the initial clinical examination. We also did not have measures of changes in nutritional status or weight over the course of treatment. Several studies have demonstrated that patients who are treated for TB often gain weight over the course of treatment [Reference van Lettow, Fawzi and Semba5], probably due to a complex relationship between lessened energy demands and increased nutritional intake when persons become more healthy [Reference Macallan4], but we were unable to document these changes or evaluate how changes in nutritional status impact outcome. Based on a previous clinical trial, failure to gain weight in the initial phase of TB treatment may increase the risk for treatment relapse [Reference Khan6]. Finally, our cohort had few patients who were confirmed as HIV positive, so we were unable to evaluate the role of HIV, one of the most important risk factors affecting TB treatment outcome, as an effect modifier or confounder in the association between nutritional status and treatment outcome.
While the current level of evidence does not support the general use of high-energy or micronutrient nutritional supplementation as adjunctive therapy to improve treatment outcome in TB patients [Reference Abba14], little is known about the utility of supplementation in specific patient groups that are malnourished or deficient in micro- or macro-nutrients at the time of TB diagnosis. There is consistent indication that supplementation may increase body weight [Reference Abba14], therefore restoring nutritional status in persons who are malnourished may improve the effectiveness of TB therapy through enhanced drug absorption and immune system function. One recent study identified that the provision of one meal of food daily throughout TB treatment resulted in a >5% weight gain after 2 months in patients that were underweight (BMI <18·5) at the time of TB diagnosis; this gain was significantly greater than gains observed in the non-intervention control group (aOR 2·69, 95% CI 1·4–5·0, P=0·002) [Reference Martins15]. However, this study, consistent with previous reports [Reference Abba14], did not identify an overall effect of the food intervention on TB treatment adherence or outcome with all patients combined (both underweight and normal weight), but suggests that such an intervention may be worth considering in patients that are malnourished [Reference Martins15].
In conclusion, MDR-TB patients who were underweight at diagnosis had a more severe clinical presentation, experienced more adverse events, and had an increased risk for death compared to patients who were of normal weight. Additional research is needed to evaluate the impact of nutritional supplementation, and specific combinations of nutrients, on TB outcome in patients who are malnourished at the time of TB or MDR-TB diagnosis. Furthermore, it is important to better understand the role of nutritional adequacy in preventing TB disease in groups at high-risk for TB infection.
ACKNOWLEDGEMENTS
Funding for this study was provided by the Latvian Ministry of Health. A small amount was contributed by the U.S. Agency for International Development to support technical assistance to the project, but they had no role in the conduct of the study. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of U.S. CDC.
DECLARATION OF INTEREST
None.