INTRODUCTION
Hepatitis B is the most common liver infection in the world [Reference Maclachlan and Cowie1, 2]. The hepatitis B virus (HBV) is transmitted through contact with infected blood and other bodily fluids and is 100 times more infectious than human immunodeficiency virus (HIV) [3]. Rate of progression from acute to chronic HBV infection varies by age and immune status at time of infection. Untreated, 20–30% of chronically infected individuals will develop liver cirrhosis or liver cancer in their lifetime [3]. In 2013, the US HBV-related mortality rate was 0·5 deaths/100 000 population, with the highest rate in Asians/Pacific Islanders (APIs) (2·6 deaths/100 000 population) [4].
The epidemiology of HBV infection varies by geographical area [Reference Maclachlan and Cowie1, Reference Stasi5]. Perinatal transmission is the most common cause of HBV infection in high endemic areas (⩾8% prevalence), such as Africa, Southeast Asia, China, and parts of South America, due to inadequate preventive measures during childbirth [Reference Stasi5, 6]. The most common form of HBV transmission in low endemic areas (<2% prevalence) is sexual transmission. The United States is considered a low endemic country with an HBV prevalence of 0·3–0·5%; however, urban centres such as New York City (NYC) experience a substantially higher burden [Reference Wasley7].
An estimated 1·3 million cases of chronic HBV were diagnosed in the United States from 1974 to 2008. The majority (95%) were foreign-born individuals who were infected in their home countries, mainly China, the Philippines, and other HBV-endemic Asian countries [Reference Mitchell8]. High rates of immigration, particularly from China, contribute to the high HBV prevalence in NYC of ~1·2%, over twice the national prevalence [Reference Wasley7, Reference France9–Reference Hutton12]. An enhanced surveillance study of 180 randomly selected newly diagnosed HBV-infected persons in NYC between 2008 and 2009 identified 67% as Asian, with 56% born in China [Reference McGibbon13]. In addition, 60–80% of liver cancer cases in APIs are associated with HBV infection [Reference Hwang14, Reference Parkin15].
Due to common routes of transmission, persons with HIV/AIDS also have a disproportionate burden of HBV infection [Reference Chun16–Reference Scharschmidt18]. NYC experiences higher rates of HIV and HBV infection than the rest of New York State or the United States as a whole, and co-infection with both viruses is associated with premature mortality (death aged <65 years) [Reference Stasi5, Reference Chun16]. Co-infection with both HIV and HBV is associated with more rapid progression of liver damage, greater levels of HBV replication and lower probability of spontaneous loss of HBV e antigen [Reference Lacombe and Rockstroh19, Reference Konopnicki20]. A meta-analysis estimated the relative risk of end-stage liver disease to be ~6·14 and cirrhosis to be 2·07 in those with HBV/HIV co-infection compared to those with HBV mono-infection [Reference Graham21].
Use of antivirals can decelerate HBV disease progression, and screening HBV-infected patients for liver cancer can reduce HBV-related mortality via early cancer diagnosis, treatment, and monitoring [Reference Moorman10, 22, 23]. Therefore, understanding the current impact of HBV on mortality at a population level will inform and improve targeted programming to strengthen HBV screening and treatment for at-risk groups. This paper describes the impact of HBV infection, with and without HIV infection (excluding those with hepatitis C virus), on mortality and examines demographic and socioeconomic risk factors for HBV-related causes of death.
METHODS
Data matching and analytical population
The NYC Department of Health and Mental Hygiene (DOHMH) implemented the Centers for Disease Control and Prevention's Program Collaboration and Service Integration initiative to increase data integration and describe interactions across infectious diseases in NYC [24]. As part of this initiative, a cross-match of disease surveillance registries was conducted, including reports of HBV, hepatitis C virus (HCV), HIV, tuberculosis, haemoglobin A1c reports, and reportable sexually transmitted diseases received during 2000–2010 and vital statistics mortality data received during 2000–2011 [Reference Drobnik25–Reference Bushnell28]. The data matching method has been described previously [Reference Pinchoff26]. Briefly, individual-level records from separate surveillance registries were linked using deterministic matching methods in SQL and SAS v. 9.2 (SAS Institute Inc., USA). Fourteen matching keys, made up of first name, last name, date of birth and social security number, were used to link the records across datasets. Datasets included all records reported between 2000 and 2011. Information on race/ethnicity, country of birth, and sex was obtained from vital statistics data for this analysis.
The analytical dataset consisted of data as described above for adults aged ⩾18 years who had an address within the 176 NYC ZIP codes at the time of their death occurring from 2000 to 2011, as identified through vital statistics data. Chronic HBV infection was classified using data from a laboratory or medical provider reported to the NYC DOHMH [29]. Individuals with a positive test for hepatitis B surface antigen (HBsAg), hepatitis B e antigen (HBeAg), HBV DNA test, or HBV genotype were considered to have HBV infection [29]. Persons with an acute HBV infection, indicated by positive hepatitis B core antibody (anti-HBc IgM), were excluded. HIV-infected individuals were defined as persons diagnosed with HIV who were alive as of 1 January 2000 and reported to the DOHMH HIV surveillance registry as of 31 December 2010. HBV/HIV co-infection was identified through the matching with the HIV surveillance registry [Reference Drobnik25]. Decedents in the dataset were identified based on matches to the vital statistics registry and restricted to persons who died with an NYC place of residence. Those with HCV infection were excluded from analyses, due to the larger prevalence of HCV and its similar impact on premature mortality and liver cancer in particular.
To explore the association between HBV-related deaths and neighbourhood characteristics, we linked each decedent's ZIP Code Tabulation Area (ZCTA) of residence at the time of death to census data [30]. ZCTAs are created by the U.S. Census Bureau to approximate ZIP codes, which are created by the U.S. Postal Service. ZIP codes that did not convert to a ZCTA, but were enclosed within a ZCTA, were modified, where appropriate [30].
Neighbourhood-level poverty, a standardized measure of socioeconomic status, was used to examine disparities in disease burden and HBV-related causes of death [Reference Toprani and Hadler31]. For deaths occurring during 2000–2004, 2005–2009, 2010, and 2011, the poverty data source was the American Community Survey (ACS), 2000, 2007–2011, 2008–2012, and 2009–2013, respectively. Categories were based on the percent of residents with household incomes below 100% of the Federal Poverty Level (FPL): low poverty (<10%), medium (10 to <20%), high (20 to <30%) and very high poverty (⩾30%) [Reference Toprani and Hadler31].
Cause of death
The DOHMH Bureau of Vital Statistics collects data on all deaths occurring in NYC. Death certificates are completed by clinicians, medical examiners, and funeral directors. The underlying cause for every death reported for NYC residents from 2000 to 2011 was grouped by ICD-10 codes into the following categories for analysis: cardiovascular-related (I00-I01, I05-I06, I09-I13, I20, I21, I24-I28, I31, I33-I35, I38, I40, I42, I44, I46, I48-I51, I60-I64, I67, I69, I70-I74, I77-I78), non-liver cancers (C00-C21, C23-C97), liver cancer (C22), liver-related such as cirrhosis and hepatitis (K70-K77, F10, B16-B19), and HIV/AIDS (B20-B24) [32]. All remaining causes of death were aggregated into ‘other’ category.
Statistical analysis
Median age at HBV report, at death, and by cause of death were compared between NYC decedents without HBV or HIV infection and those with HBV mono-infection, and between those with HBV mono-infection and HBV/HIV co-infection, using the Wilcoxon Signed-Rank test. χ 2 tests were used to compare the proportion of those with or without HBV report who died prematurely (defined as death at age <65 years) or very prematurely (defined as death at age <50 years). Demographic factors were examined. To assess the relationship between HBV status and specific causes of death, we constructed multivariable logistic regression models using a binary outcome variable for each category of death. Decedents with HIV mono-infection were excluded from the logistic regression models because premature death from HIV/AIDS tends to occur at a younger age than HBV-related causes of death, and HBV-related mortality was the primary outcome of interest. All models controlled for sex, age at death (categorized as <50, 50–64 and ⩾65 years), year of death, race/ethnicity, foreign-born status, and neighbourhood poverty level. Statistical analyses were conducted using SAS. For all analyses, we considered P < 0·05 to indicate statistical significance.
Four maps were created to assess geographical distribution of poverty, proportion of ZCTA populations born in China, and average age-adjusted HBV-related mortality by ZCTA. NYC DOHMH population estimates by age group and poverty level were obtained from U.S. Census Bureau interpolated intercensal population estimates from 2000 to 2011. We calculated annual mortality rates per ZCTA using the average number of deaths per year as the numerator and the average population size for each year between 2000 and 2011 in each ZCTA. Mortality rates were adjusted using direct standardization for age at death, grouped into 10 age groups (<1, 1–14, 15–24, 25–34, 35–44, 45–54, 55–64, 65–74, 75–84, ⩾85), and weighted by the US 2000 standard population. The neighbourhood poverty map and percent Chinese-born maps were based on 2010 estimates from the 2010 ACS [33]. Maps were generated using ArcGIS, v. 10.2 [Environmental Systems Research Institute (ESRI) 2011; ArcGIS Desktop, Release 10, USA].
RESULTS
During 2000–2011, 588 346 persons aged ⩾18 years died in NYC. A total of 23 980 (4·1%) persons had HCV, and were excluded. Based on match results, after excluding HCV infections (including 56 with HCV/HBV co-infection), a total of 568 753 (97%) had neither HIV nor HBV infection, 15 247 (2·6%) were HIV mono-infected, and 3272 (0·6%) were HBV mono-infected. Of those with HBV, 1074 (25%) were co-infected with HIV.
Demographics
The majority of HBV mono-infected (68%), HIV mono-infected (65%) and HBV/HIV co-infected (77%) decedents were male compared to 46% of decedents with neither infection (Table 1). Of HBV mono-infected decedents, 20% were non-Hispanic white compared to 54% of uninfected decedents. In contrast, 37% of HBV mono-infected decedents were API compared to only 1% of HBV/HIV co-infected decedents and 5% of those with neither infection. Of APIs, 99% were foreign-born, and of the foreign-born APIs, 74% were born in China. In contrast, the majority (67%) of HBV/HIV co-infected decedents were non-Hispanic black. Of HIV mono-infected decedents, 55% were non-Hispanic black, and 1% were API. Fifty-four percent of those who died in NYC with neither infection were white, 25% were non-Hispanic black, and 5% were APIs.
Table 1. Demographic characteristics of decedents aged ⩾18 years, New York City, 2000–2011
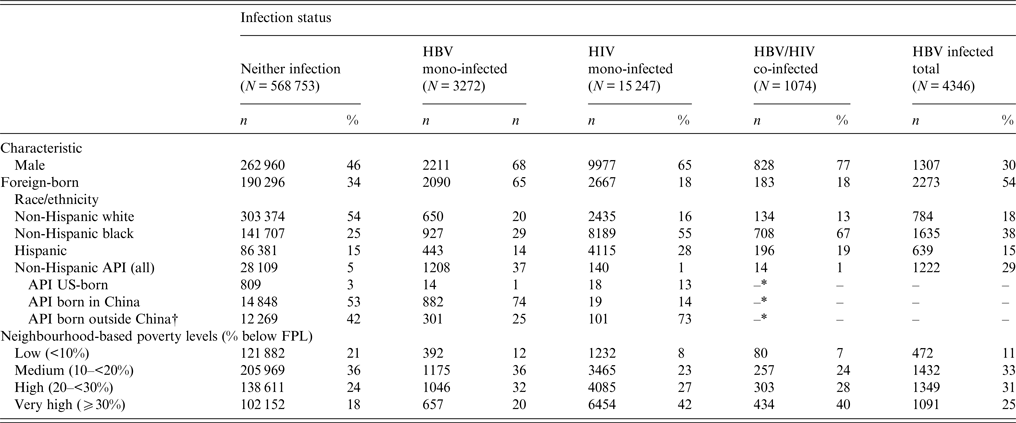
API, Asian/Pacific Islander; FPL, Federal Poverty Level.
* Cells representing ⩽15 person(s) with an underlying denominator of ⩽500 persons were suppressed.
† Highest proportions contributed by: Korean (6·2%), Hong Kong (3·1%), Philippines (2·5%) and Bangladesh (2·4%)
Socioeconomic disparities were observed when comparing neighbourhood poverty levels at time of death by infection status. Eighteen percent of uninfected decedents and 20% of HBV mono-infected individuals lived in a very high poverty (⩾30% below FPL) area at the time of death. In contrast, nearly half of HIV mono-infected (42%) and HBV/HIV co-infected (40%) decedents lived in a very high poverty area.
Premature death
Compared with New Yorkers with neither infection, the proportions of premature and very premature deaths were significantly higher in HBV mono-infected, HIV mono-infected, and HBV/HIV co-infected decedents (Fig. 1). Of HBV mono-infected decedents, 1803 (55%) died prematurely, and a subset of 706 (21%) died very prematurely. Of HBV/HIV co-infected decedents, 1060 (95%) died prematurely, and a subset of 767 (69%) died very prematurely. These proportions were significantly higher than those observed in HIV mono-infected decedents (92% and 54%, respectively, P < 0·001 for each).

Fig. 1. Proportion of deaths that were not premature, premature (50–64 years) or very premature (<50 years) in New York City, stratified by report of HBV, 2000–2011. *Statistical significance (P < 0·05) when compared to decedents with neither infection.
The median age at HBV report was significantly younger for HBV/HIV co-infected decedents compared to HBV mono-infected decedents [43 years, interquartile range (IQR) 38–50 vs. 60 years, IQR 49–72) (Table 2). The median age at death for HBV/HIV co-infected was 46 (IQR 40–52), similar to HIV mono-infected (48, IQR 41–56), and significantly younger compared to HBV mono-infected (63, IQR 52–75) decedents and with New Yorkers with neither infection (79, IQR 66–87) (Table 2).
Table 2. Median age at first report of HBV, age at death by infection status, New York City, 2000–2011
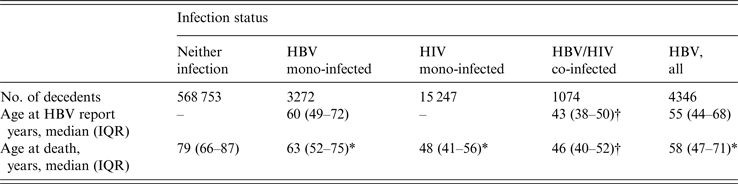
IQR, Interquartile range.
* Statistical significance (P < 0·05) compared with decedents with neither infection.
† Statistical significance (P < 0·05) compared with decedents with HBV mono-infection.
Causes of death
In NYC decedents with neither HBV nor HIV infection, the leading causes of death were cardiovascular causes (48%) and non-liver cancers (23%), with only 1% dying of liver cancer (Table 3). Of decedents with HBV mono-infection, 27% died from cardiovascular-related causes, 25% from non-liver cancer, and 15% from liver cancer. Of decedents with HIV mono-infection, 62% died from HIV/AIDS and 1% died of liver cancer. For decedents with HBV/HIV co-infection, 66% of deaths were due to HIV/AIDS and 3% were due to liver cancer. The median age at death was significantly younger for those with HBV mono-infection compared to those with neither infection in those who died of cardiovascular causes (70 years vs. 82 years), non-liver cancers (62 years vs. 73 years), and liver cancer (58 years vs. 72 years).
Table 3. Number of decedents and median age at death stratified by cause of death and infection status
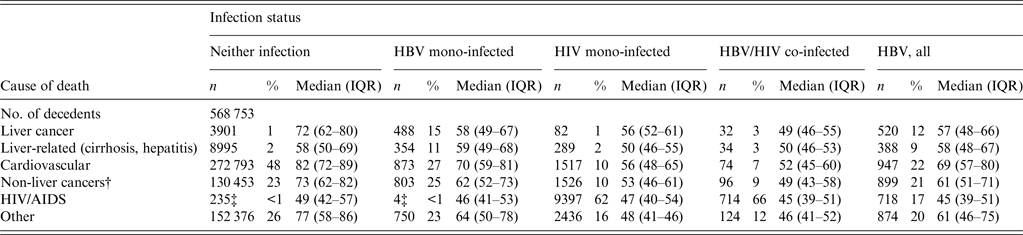
IQR, Interquartile range.
† Predominantly lung, colon, pancreas, and stomach cancers.
‡ HIV/AIDS deaths in persons not identified by HIV surveillance at time of death.
The adjusted odds for each cause of death were compared for HBV mono-infected decedents compared to decedents with neither infection, and HIV mono-infected decedents compared to HBV/HIV co-infected decedents (Table 4). All models controlled for sex, age at death, year of death, race/ethnicity, foreign-born status, and neighbourhood level poverty. Compared to decedents with neither infection, the odds of dying of liver cancer in HBV mono-infected individuals were almost 15 times higher [odds ratio (OR) 14·96, 95% confidence interval (CI) 13·33–16·71] and the odds of dying of liver-related non-cancer causes were about six times higher (OR 6·03, 95% CI 5·34–6·84). Compared to HIV mono-infected decedents, HBV/HIV co-infected decedents had over six times higher odds of dying of liver cancer (OR 6·20, 95% 3·97–9·68), and over two times higher odds of dying of liver-related non-cancer causes of death (OR 2·12, 95% CI 1·45–3·09). Compared to uninfected decedents, HBV/HIV co-infected decedents had almost five times the odds of dying of liver cancer (OR 4·57, 95% CI 3·15–6·64). However, the majority of decedents with HIV mono-infection and HBV/HIV co-infection died of HIV/AIDS (62% and 66%, respectively).
Table 4. Associations between cause-specific deaths and infection status in New York City, 2000–2011

OR, Odds ratio; CI, confidence interval.
Controlling for sex, age at death, year of death, race/ethnicity, foreign-born status, and ZCTA-level poverty.
Geographical patterns
Because the majority of foreign-born decedents with HBV mono-infection were from China, we examined whether this sub-population was also concentrated in one of NYC's distinct Chinese neighbourhoods. The average age-adjusted mortality rate in HBV mono-infected persons per ZCTA and the proportion of persons born in China by ZCTA are depicted in Figure 2(a, b). Forty percent of HBV mono-infected decedents resided in one of ten ZCTAs with the highest proportion of persons born in China (>25% population), corresponding with NYC's three Chinatown neighbourhoods.

Fig. 2. (a) Average age-standardized mortality rate in HBV mono-infected persons/100 000 from 2000 to 2011, (b) percentage Chinese-born in 2010, (c) average age-standardized mortality rate in HBV/HIV co-infected persons per 100 000 from 2000–2011, and (d) poverty category per modified ZCTA in 2010, New York City. * Locator Map [Department of Epidemiology Services, New York City Department of Health. NYC Borough Map (https://www1.nyc.gov/assets/doh/downloads/pdf/epi/NYC_Borough_Map.pdf)].
The average mortality rate in HBV/HIV co-infected persons and poverty levels per ZCTA are depicted in Figure 2(c, d). Overall, areas with high mortality rates in HBV/HIV co-infected persons (Fig. 2c ) did not overlap with areas exhibiting high mortality rates in HBV mono-infected (Fig. 2a ). Rather, areas with high HBV/HIV co-infection mortality rates were concentrated in ZCTAs with high and very high poverty, mainly in northern Manhattan and the Bronx (Fig. 2d ).
DISCUSSION
We identified a disproportionate burden of premature death, particularly due to liver cancer, in individuals with HBV in NYC. Mono-infection with HBV was associated with nearly 15-fold higher odds of liver cancer-associated mortality compared to neither infection. In contrast, HBV/HIV co-infected individuals were more likely to die at younger ages and from other causes; 66% of the co-infected decedents died of HIV/AIDS-related causes. However, HBV co-infection appeared to further increase the likelihood of premature death in those with HIV; of these co-infected decedents, risk of dying of liver cancer was increased by over six times. The association between premature death and HBV infection in NYC suggests the need for improved delivery of prevention and care for both persons with and without concurrent HIV infection.
Persons born in countries with intermediate (2–8%) or high (>8%) prevalence should be screened for HBV and, if negative, vaccinated against HBV [22, Reference Weinbaum34]. Global vaccination rates have increased recently to 82%, and 184 countries now offer HBV vaccination, suggesting the burden of HBV imported into NYC may decline in the future [35]. However, immigrants from endemic countries are often already HBV-infected before entering the United States, and many immigrant APIs remain unvaccinated and unaware that HBV is vaccine-preventable [Reference Stasi5, Reference Wasley7, Reference Weinbaum34].
Our analysis identified NYC neighbourhoods where screening, prevention and linkage to care campaigns need to be targeted. In NYC, 37% of HBV mono-infected decedents were APIs, of whom 74% were born in China, a country with very high HBV prevalence. In addition, 40% of HBV mono-infected decedents were geographically clustered and resided in one of NYC's three Chinatown neighbourhoods. Prioritizing these high prevalence neighbourhoods with culturally and linguistically tailored educational campaigns and educating clinicians and healthcare providers in high-risk communities on HBV screening recommendations may detect infection earlier and reduce HBV-associated mortality [Reference Chao36].
Despite evidence-based interventions and practice guidelines, HBV mono-infected adults may face barriers to care due to immigration status, lack of health insurance, stigma, language barriers, or lack of awareness [Reference Hwang14, Reference Weinbaum34, Reference Chao, Chang and So37, Reference Juon38]. Many HBV-infected individuals are unaware of their infection status and, consequently, do not seek treatment [Reference Hwang14, Reference Chao, Chang and So37, Reference Juon38]. Lack of education regarding HBV transmission and symptoms are also prevalent in the API population and potential misconceptions and stigma regarding HBV infection hinder HBV test-seeking behaviour [6, Reference Chao, Chang and So37]. An enduring barrier to care is lack of health insurance for persons with undocumented immigration status; improving health insurance options and access to healthcare for immigrants regardless of their immigration status may improve health outcomes and prevent new infections in this population.
Twenty-five percent of HBV-infected decedents in NYC also had HIV, a common co-infection due to shared risk factors [23]. Co-infection with HIV alters the natural history of HBV and vice versa, leading to more rapid progression of disease. For example, concurrent HBV infection increases the risk of drug-related hepatoxicity and reduces the effectiveness of highly active antiretroviral therapy in HIV-infected individuals [Reference Sulkowski39, Reference Núñez40]. Increased levels of HBV replication associated with HIV infection, accompanied by significantly lower rates of spontaneous reduction in viral replication have also been observed in co-infected individuals [Reference Hoffman and Thio41, Reference Oshitani42].
HBV/HIV co-infected individuals were at disproportionate risk for very premature death; of all HBV/HIV co-infected decedents, 95% died prematurely and 69% died very prematurely, proportions that were higher than those with HIV mono-infection. The primary cause of death in co-infected individuals was HIV/AIDS. However, findings suggest that co-infection with HBV may lead to worse HBV outcomes such as liver cancer, as 3% of co-infected deaths were due to liver cancer compared to 1% of HIV mono-infected deaths. After controlling for various demographic factors in multivariable logistic regression models, HBV/HIV co-infected adults still had over six times higher odds of dying of liver cancer compared to those with HIV only. This finding is consistent with literature that suggests co-infection with HBV and HIV leads to worse outcomes [Reference Scharschmidt18, Reference Lacombe and Rockstroh19].
HBV/HIV co-infected decedents in NYC were also geographically and demographically distinct from HBV mono-infected decedents. Co-infected decedents were disproportionately non-Hispanic black (67%) and resided in very high poverty neighbourhoods (40%) in northern Manhattan and the Bronx at the time of death. They were also less likely to be foreign-born (18%) than HBV mono-infected individuals (65%). These patterns follow those for HIV mono-infection, which disproportionately impacts high poverty neighbourhoods. These key demographic differences indicate the need for tailored interventions and messaging.
To prevent HBV infection in people with HIV, efforts to provide HBV vaccination and counselling on reducing transmission risk are recommended and should be strengthened [Reference Weinbaum34, 43]. Additionally, early initiation of antiretroviral therapy has been shown to decrease HBV-associated mortality and sequelae in HBV/HIV co-infected persons [Reference Bräu44]. Robust implementation of HIV testing, prevention, and linkage to care programmes can also prevent HIV-related and HBV-related mortality in persons with or at higher risk for HBV co-infection, such as people who inject drugs and men who have sex with men [Reference Lacombe and Rockstroh19, Reference Bräu44].
Cardiovascular-related and non-liver cancers were the leading causes of death in New Yorkers with neither HBV nor HIV infection and with HBV mono-infection. However, 15% of HBV mono-infected individuals died of liver cancer, significantly higher than New Yorkers without HBV (1%). With antiviral therapy and liver cancer screening, studies suggest that these HBV-attributed deaths can be reduced [Reference Stasi5, Reference Moorman10, Reference Lacombe and Rockstroh19, Reference Juon38, Reference Bräu44].
Strengths and limitations
Strengths of this study included the use of novel data-matching methods to understand the burden of co-infections. Chronic HBV, HIV, and vital statistics registries in NYC were matched, allowing for comparison and analysis of mortality outcomes and specific causes of death for HBV-infected individuals. This is a large, comprehensive dataset of all reported HBV infections in NYC, a major urban centre with a disproportionately high burden of HBV. The matching of these data allows for a unique citywide, population-level analysis that has not been conducted previously. Describing the two distinct populations impacted by HBV will inform citywide HBV prevention and management policy.
This study also has some limitations. Denominators were not available for all demographic and risk groups so that subgroup mortality rates could not be calculated and compared. Errors in death certificates may be present in the data, such as misclassification of underlying cause of death, or biases toward specific causes of death (e.g. HIV/AIDS is always categorized as the underlying cause of death in HIV-infected decedents), may also be present in the data [45]. Vital statistics data are limited to deaths recorded within NYC, and NYC residents who died outside NYC were not captured in this study [Reference Drobnik25]. Additionally, some individuals in the chronic HBV dataset may have had acute infection and were no longer infected or may have had a false-positive test result. Persons undiagnosed or unreported with HBV or HIV were misclassified as having neither infection, although laboratory reporting on positive diagnostics should be very complete.
CONCLUSIONS
This study is the first population-level analysis of cause-specific mortality and HBV in NYC. Chinese immigrants and HIV-infected persons need easily accessible services for HBV screening, medical management, early initiation of antiviral or antiretroviral therapy, and liver cancer screening [Reference Stasi5, Reference Huang11, Reference Kumar, Singla and Kacharya46]. Through these efforts, incidence of HBV infection (in persons with HIV), liver cancer (mainly in HBV mono-infected persons), premature mortality, and exacerbated HIV disease progression may be decreased [Reference Lacombe and Rockstroh19, Reference Drobnik25, Reference Kumar, Singla and Kacharya46].
ACKNOWLEDGEMENTS
The authors thank Sonny Ly and Julie Yuan for conducting the data match, Sharon Greene for her epidemiological guidance, and Wenhui Li for his assistance with vital statistics data. The authors also thank Sarah Braunstein, James Hadler, Fabienne Laraque, Gretchen Van Wye, and Jay K. Varma for their comments on the manuscript.
DECLARATION OF INTEREST
None.









